C. Perfringens Neuraminidase / NA (Active) (His Tag)
NA
- 100ug (NPP3150) Please inquiry
| Catalog Number | P11680-V07E |
|---|---|
| Organism Species | C.perfringens |
| Host | E. coli |
| Synonyms | NA |
| Molecular Weight | The recombinant C. Perfringens neuraminidase consisting of 388 amino acids and has a calculated molecular mass of 43.6 kDa. It migrates as an approximately 40 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 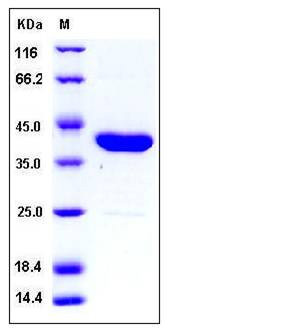 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the C. Perfringens neuraminidase (P10481.1) (Cys 2-Gln 382) was expressed, with a N-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to cleave a fluorogenic substrate, 2'-(4-Methylumbelliferyl)-α-D-N-acetylneuraminic acid. The specific activity is 150,000 pmoles/min/μg (Activity of this protein is much better than the world's famous brand) as measured under the described conditions . |
| Research Area | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Clostridium perfringens / C. perfringens (formerly known as C. welchii) is a Gram-positive, rod-shaped, anaerobic, spore-forming bacterium of the genus Clostridium. C. perfringens is ubiquitous in nature and can be found as a normal component of decaying vegetation, marine sediment, the intestinal tract of humans and other vertebrates, insects, and soil. C. perfringens is commonly encountered in infections as a benign component of the normal flora. In this case, its role in disease is minor. Infections due to C. perfringens show evidence of tissue necrosis, bacteremia, emphysematous cholecystitis, and gas gangrene, which is also known as clostridial myonecrosis. NA, also called sialidases, specifically catalyze the hydrolysis removal of terminal sialic acid residues from viral and cellular glycoconjugates. C. Perfringens neuraminidase catalyzes the hydrolysis of alpha-(2->3)-, alpha-(2->6)-, glycosidic linkages of terminal sialic acid residues in oligosaccharides, glycoproteins, glycolipids, colominic acid and synthetic substrates, but has little activity against the α2-8 glycosidic linkages. The function of the neuraminidase is to release sialic acids for use as carbon and energy sources for the non-pathogenic bacterium, while in pathogenic microorganisms, sialidases have been suggested to be pathogenic factors |
| Reference |
