Cynomolgus / Rhesus c-MET / HGFR Protein
MET
- 100ug (NPP1098) Please inquiry
| Catalog Number | P90304-CCCH |
|---|---|
| Organism Species | Cynomolgus |
| Host | Human Cells |
| Synonyms | MET |
| Molecular Weight | The recombinant cynomolgus/rhesus MET comprises 914 amino acids and has a calculated molecular mass of 102.3 KDa. The apparent molecular mass of it is approximately 73-93 KDa in SDS-PAGE under reducing conditions. |
| predicted N | Glu 25 |
| SDS-PAGE | 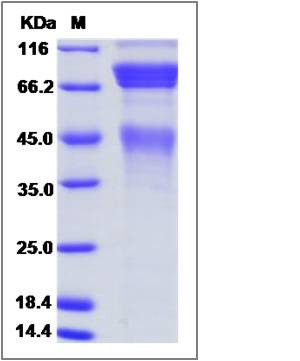 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the cynomolgus/rhesus MET (NP_001162100.1) (Met1-Thr932) was expressed five amino acids (DDDDK) the C-terminus. Cynomolgus and Rhesus MET sequences are identical. |
| Bio-activity | Immobilized Cynomolgus MET at 10 μg/ml (100 μl/well) can bind biotinylated Cynomolgus HGF (cat:90286-CNAH), The EC50 of biotinylated Cynomolgus HGF (cat:90286-CNAH) is 0.17-0.41 μg/ml. |
| Research Area | Cancer |Signal transduction |Protein Phosphorylation |Tyrosine Kinase |Receptor Tyrosine Kinases |
| Formulation | Lyophilized from sterile PBS, pH 7.4. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Hepatocyte growth factor receptor (HGFR), also known as c-Met or mesenchymal-epithelial transition factor (MET), is a receptor tyrosine kinase (RTK) that has been shown to be overexpressed and/or mutated in a variety of malignancies. HGFR protein is produced as a single-chain precursor, and HGF is the only known ligand. Normal HGF/HGFR signaling is essential for embryonic development, tissue repair or wound healing, whereas aberrantly active HGFR has been strongly implicated in tumorigenesis, particularly in the development of invasive and metastatic phenotypes. HGFR protein is a multifaceted regulator of growth, motility, and invasion, and is normally expressed by cells of epithelial origin. Preclinical studies suggest that targeting aberrant HGFR signaling could be an attractive therapy in cancer. |
| Reference |
