Cynomolgus SerpinC1 / AntithrombinIII / ATIII Protein (His Tag)
SerpinC1
- 100ug (NPP4446) Please inquiry
| Catalog Number | P90042-C08H |
|---|---|
| Organism Species | Cynomolgus |
| Host | Human Cells |
| Synonyms | SerpinC1 |
| Molecular Weight | The recombinant cynomolgus SerpinC1 consists of 441 amino acids and has a calculated molecular mass of 50.2 kDa. The apparent molecular mass of the recombinant protein is approximately 55-60 kDa in SDS-PAGE under reducing conditions due to glycosylation. |
| predicted N | Ser 35 |
| SDS-PAGE | 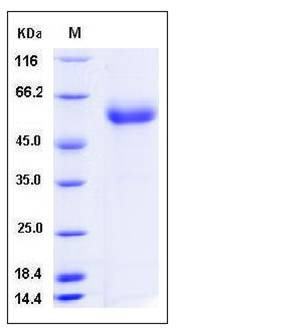 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the Cynomolgus (Macaca fascicularis) SerpinC1 (Met 1-Ser 464) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | Measured by its ability to inhibit thrombin cleavage of a fluorogenic peptide substrate Boc-VPR-AMC, R&D Systems, Catalog# ES011. The IC50 value is <5 nM. |
| Research Area | Cardiovascular |Blood |Coagulation |Coagulation Cascade |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | SerpinC1, also known as antithrombin III (AT III), is a member of the serpin superfamily of serine protease inhibitors, and has been found to be a marker for disseminated intravascular coagulation (DIC) and to be of prognostic significance in septic patients. SerpinC1 synthesized in the liver is the principal plasma serpin of blood coagulation proteases and inhibits thrombin and other factors such as Xa by the formation of covalently linked complexes. Thus it is one of the most important coagulation inhibitors and the fundamental enzyme for the therapeutical action of heparin. In common with SerpinA5 and D1, the inhibitory activity of SerpinC1 undergoes a dramatic increase in the presence of heparin and other glycosaminoglycans. ATIII mediates the promotion of prostaglandin release, an inhibitor of leucocyte activation and downregulator of many proinflammatory cytokines. Antithrombin III exerts anti-inflammatory properties in addition to its anti-coagulative mechanisms. In animal models of sepsis, ATIII affected cytokine plasma concentrations with a decrease of pro-inflammatory cytokines. The deficiency or functional abnormality of ATIII may result in an increased risk of thromboembolic disease, such as deep vein thrombosis and pulmonary embolism. In addition, it has been reported that SerpinC1 can alter or influence inflammatory processes via inhibition of NF-κB activation or actin polymerization. |
| Reference |
