Escherichia coli acnB Protein
yacI,yacJ
- 100ug (NPP3153) Please inquiry
| Catalog Number | P13102-ENAE |
|---|---|
| Organism Species | E. coli |
| Host | E. coli |
| Synonyms | yacI,yacJ |
| Molecular Weight | The recombinant E.Coli acnB consisting of 865 amino acids and has a calculated molecular mass of 93.5 kDa. It migrates as an approximately 95 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met 1 |
| SDS-PAGE | 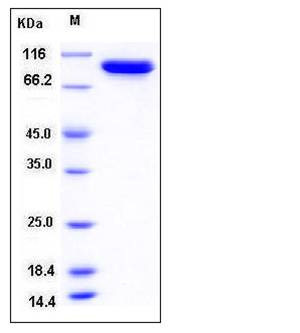 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the E.Coli (strain K12) acnB (P36683) (Met 1-Val 865) was expressed and purified. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile 40mM Tris, 1mM DTT, pH 8.2 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Escherichia coli contains two major aconitases (Acns), AcnA and AcnB. They are distantly related monomeric Fe-S proteins that contain different arrangements of four structural domains. acnA is specifically subject to SoxRS-mediated activation, whereas acnB encodes the major aconitase that is synthesized earlier in the growth cycle than AcnA. It is concluded that AcnB is the major citric acid cycle enzyme. Aconitate hydratase 2 (acnB) catalyzes the isomerization of citrate to isocitrate via cis-aconitate as well as the dehydration of 2-methylisocitrate to cis-2-methylaconitate, thus it functions as the major citric-acid-cycle enzyme during exponential growth. Escherichia coli acnB serves as either an enzymic catalyst or a mRNA-binding post-transcriptional regulator, depending on the status of its iron sulfur cluster. AcnB represents a large, distinct group of Gram-negative bacterial aconitases that have an altered domain organization relative to mitochondrial aconitase and other aconitases. |
| Reference |
