Human ACE2 / Angiotensin-Converting Enzyme 2 Protein (Fc Tag)
ACEH
- 100ug (NPP3553) Please inquiry
| Catalog Number | P10108-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | ACEH |
| Molecular Weight | The recombinant human ACE2/Fc is a disulfide-linked homodimeric protein. The reduced monomer consists of 961 amino acids and predicts a molecular mass of 110.3 kDa. As a result of glycosylation, the rhACE2/Fc monomer migrates as approximately 145-150 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Gln 38 |
| SDS-PAGE | 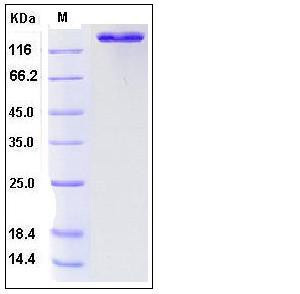 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Ser 740) of human ACE2 precursor (NP_068576.1) was expressed with the fused Fc region of human IgG1 at the C-terminus. |
| Bio-activity | 1. Using the Octet RED System, the affinity constant (Kd) of human AEC2-Fc (P 10108-H02H) bound to Spike (HCoV-EMC/2012) (P 40071-V31B) was 6 nM. 2. Using the Octet RED System, the affinity constant (Kd) of human AEC2-Fc (P 10108-H02H) bound to Spike (HCoV-EMC/2012) (P 40071-V05B) was 30 nM. 3. Using the Octet RED System, the affinity constant (Kd) of human AEC2-Fc (P 10108-H02H) bound to Spike (HCoV-EMC/2012) (ECD, aa 1-1297) (P 40069-V08B) was 30 nM. 4. Using the Octet RED System, the affinity constant (Kd) of human AEC2-Fc (P 10108-H02H) bound to Spike-His (aa 1-760) (P 40021-V08H) was 10 nM. |
| Research Area | Microbiology |Pathogenic microorganism |viruses |animal virus |Virus infection associated |Virus host receptor | |
| Formulation | Lyophilized from sterile 100mM Glycine, 10mM NaCl, 50mM Tris, pH 7.5 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Angiotensin-converting enzyme 2 (ACE2), a first homolog of ACE, regulates the renin angiotensin system (RAS) by counterbalancing ACE activity. Accumulating evidence in recent years has demonstrated a physiological and pathological role of ACE2 in the cardiovascular, renal and respiratory systems. ACE2 also has an important role in blood pressure control. This enzyme, an homolog of ACE, hydrolyzes angiotensin (Ang) I to produce Ang-(1-9), which is subsequently converted into Ang-(1-7) by a neutral endopeptidase and ACE. ACE2 releases Ang-(1-7) more efficiently than its catalysis of Ang-(1-9) by cleavage of Pro(7)-Phe(8) bound in Ang II. Thus, the major biologically active product of ACE2 is Ang-(1-7), which is considered to be a beneficial peptide of the RAS cascade in the cardiovascular system. A physiological role for ACE2 has been implicated in hypertension, cardiac function, heart function and diabetes, and as a receptor of the severe acute respiratory syndrome coronavirus. In the acute respiratory distress syndrome (ARDS), ACE, AngII, and AT1R promote the disease pathogenesis, whereas ACE2 and the AT2R protect from ARDS. Importantly, ACE2 has been identified as a key SARS-coronavirus receptor and plays a protective role in severe acute respiratory syndrome (SARS) pathogenesis. Furthermore, the recent explosion of research into the ACE2 homolog, collectrin, has revealed a new physiological function of ACE2 as an amino acid transporter, which explains the pathogenic role of gene mutations in Hartnup disorder. This review summarizes and discusses the recently unveiled roles for ACE2 in disease pathogenesis. |
| Reference |
