Human ACO1 / irp1 Protein (His Tag)
ACONS,HEL60,IREB1,IREBP,IREBP1,IRP1
- 100ug (NPP1861) Please inquiry
| Catalog Number | P10966-H07B |
|---|---|
| Organism Species | Human |
| Host | Baculovirus-Insect Cells |
| Synonyms | ACONS,HEL60,IREB1,IREBP,IREBP1,IRP1 |
| Molecular Weight | The recombinant human ACO1 consists of 908 amino acids and has a calculated molecular mass of 101 kDa. It migrates as an approximately 90 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 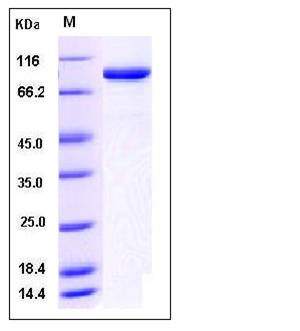 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human ACO1 (P21399) (Met 1-Lys 889) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Metabolism |Pathways and Processes |Vitamins / minerals |
| Formulation | Lyophilized from sterile 50mM Tris, 100mM NaCl, pH 8.0, 10% gly, 2mM DTT 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Aconitase 1(ACO1) or IRP1 is one member of the aconitase family that contains a diverse group of iron-sulphur(Fe-S) isomerases and two types of iron regulatory protein. Aconitase exits in two forms: one is soluble and the other is mitochondrial. ACO1 is the soluble existing form, and the mitochondrial form is ACO2. Residues from all three N-terminal domains and the larger C-terminal domain contribute to the active site region. When the enzyme is activated, it gains an additional iron atom. ACO1 can assume two different functions in cells, depending on different conditions. During iron scarcity or oxidative stress, ACO1 binds to mRNA stem-loop structures called iron responsive elements to modulate the translation of iron metabolism genes. In iron-rich conditions, ACO1 binds an iron-sulfur cluster to function as a cytosolic aconitase. |
| Reference |
