Human ACVR2B / ActivinR-IIB Protein (His Tag)
Activin RIIB,ActR-IIB,ACTRIIB,HTX4
- 100ug (NPP1047) Please inquiry
| Catalog Number | P10229-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | Activin RIIB,ActR-IIB,ACTRIIB,HTX4 |
| Molecular Weight | The recombinant human ACVR2B comprises 127 amino acids and predicts a molecular mass of 15 kDa. As a result of glycosylation, rh ACVR2B migrates as an approximately 33-38 kDa protein in SDS-PAGE under reducing conditions. |
| predicted N | Ser 19 |
| SDS-PAGE | 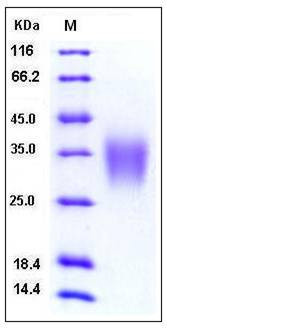 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain of human ACVR2B (NP_001097.2) (Met 1-Thr 134) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | 1. Measured by its ability to bind biotinylated Human INHBA-his (P10429-H08H)in functional ELISA. 2. Measured by its ability to bind biotinylated Mouse INHBA-his (P50659-M08H) in functional ELISA. 3. Measured by its ability to neutralize Activin-mediated inhibition on MPC11 cell proliferation. The ED50 for this effect is typically 0.3-2 µg/mL in the presence of 10 ng/mL recombinant Activin A. |
| Research Area | Cancer |Signal transduction |Cytoskeleton / ECM |Cytoskeletal Proteins |Microfilaments |Actin etc | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | ACVR2A and ACVR2B are two activin type II receptors. ACVR2B is integral to the activin and myostatin signaling pathway. Ligands such as activin and myostatin bind to ACVR2A and ACVR2B. Myostatin, a negative regulator of skeletal muscle growth, is regarded as a potential therapeutic target and binds to ACVR2B effectively, and to a lesser extent, to ACVR2A. The structure of human ACVR2B kinase domain in complex with adenine establishes the conserved bilobal architecture consistent with all other catalytic kinase domains. Haplotype structure at the ACVR2B and follistatin loci may contribute to interindividual variation in skeletal muscle mass and strength. Defects in ACVR2B are a cause of left-right axis malformations. |
| Reference |
|
