Human ADAM12 Protein (His Tag)
ADAM12-OT1,CAR10,MCMP,MCMPMltna,MLTN,MLTNA
- 100ug (NPP3558) Please inquiry
| Catalog Number | P10896-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | ADAM12-OT1,CAR10,MCMP,MCMPMltna,MLTN,MLTNA |
| Molecular Weight | The recombinant human ADAM12 consists of 496 amino acids and predicts a molecular mass of 55.2 kDa. As a result of glycosylation, rhADAM12 migrates as three bands with apparent molecular mass of 27, 55 and 72 kDa corresponding to the propeptide, the mature form and the pro form respectively in SDS-PAGE under reducing conditions. |
| predicted N | Arg 29 |
| SDS-PAGE | 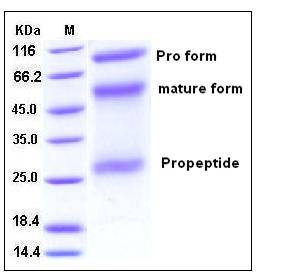 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human ADAM12 isoform 1 (NP_003465.3) extracellular domain (Met 1-Ser 513) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | Measured by its binding ability in a functional ELISA. Immobilized human ADAM12-His (P 10896-H08H) at 10 μg/ml (100 μl/well) can bind biotinylated mouse FLRG-His (P 10591-M08H) with a linear range of 0.31-1.25 μg/ml. |
| Research Area | Developmental Biology |Metabolism |Pathways and Processes |Metabolism processes |Apoptosis |Integrin | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | The ADAMs (a disintegrin and metalloprotease) comprise a family of multidomain proteins with metalloprotease, cell adhesion, and signaling activities. Human ADAM12, which is implicated in diseases such as cancer, is expressed in two splice forms, the transmembrane ADAM12-L and the shorter and soluble ADAM12-S. ADAM12, also known as and Meltrin alpha, is a member of the ADAM protein family, which contains one disintegrin domain, one EGF-like domain and one peptidase M12B domain. ADAM12 is synthesized as a zymogen with the prodomain keeping the metalloprotease inactive through a cysteine-switch mechanism. Maturation and activation of the protease involves the cleavage of the prodomain in the trans-Golgi or possibly at the cell surface by a furin-peptidase. It is a membrane-anchored metalloprotease, which has been implicated in activation-inactivation of growth factors that play an important role in wound healing, including heparin-binding epidermal growth factor (EGF)-like growth factor (HB-EGF) and IGF binding proteins. ADAM12 may also regulate cell-cell and cell-extracellular matrix contacts through interactions with cell surface receptors - integrins and syndecans - potentially influencing the actin cytoskeleton. Moreover, ADAM12 interacts with several cytoplasmic signaling and adaptor molecules through its intracellular domain, thereby directly transmitting signals to or from the cell interior. These ADAM12-mediated cellular effects appear to be critical events in both biological and pathological processes. In addition to protease activity, ADAM12 possesses cell binding and cell signaling properties. In many studies, ADAM12 overexpression has been correlated with disease, and ADAM12 has been shown to promote tumor growth and progression in cancer. On the other hand, protective effects of ADAM12 in disease have also been reported. |
| Reference |
