Human ADAM15 / MDC15 Protein (His Tag)
MDC15
- 100ug (NPP3559) Please inquiry
| Catalog Number | P10517-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | MDC15 |
| Molecular Weight | The secreted recombinant human ADAM15 comprises 501 amino acids after proteolytic of the signal peptide and pro peptide and has a predicted molecular mass of 54 kDa. As a result of glycosylation, rh ADAM15 migrates as an approximately 65-70 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Asp 207 |
| SDS-PAGE | 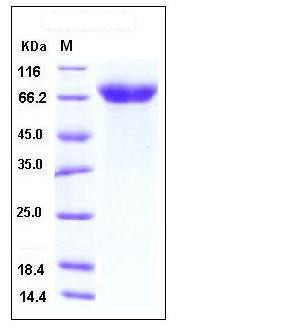 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human ADAM15 (NP_997074.1) (Met 1-Thr 696) precursor with a C-terminal polyhistidine tag was expressed. |
| Bio-activity | |
| Research Area | Developmental Biology |Morphogenesis |Hedgehog Signaling Pathway |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | ADAM15, also known as Metargidin, is a type I transmembrane glycoprotein belonging to the ADAM (A Disintegrin and Metalloprotease Domain) family of proteins and is widely expressed in different tissues and cell types. Members of this family contain an amino-terminal metalloprotease domain followed by a disintegrin domain, a cysteine-rich region and a membrane proximal EGF-like domain. The disintegrin domain of ADAM15/metargidin contains an RGD tripeptide sequence, suggesting that it may potentially interact with the integrin family of proteins. ADAM15 is a transmembrane multi-domain proteins implicated in proteolysis, cell-cell and cell-matrix interactions in various disease conditions. There is also evidence supporting a role for ADAM15 in angiogenesis and angioinvasion of tumor cells, which are critical for unrestrained tumor growth and metastatic spread. Given its diverse functions, ADAM15 may represent a pivotal regulatory component of tumor progression, an important target for therapeutic intervention, or emerge as a biomarker of disease progression. |
| Reference |
