Human AGER / RAGE Protein (Fc Tag)
RAGE
- 100ug (NPP4210) Please inquiry
| Catalog Number | P11629-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | RAGE |
| Molecular Weight | The recombinant human AGER/Fc chimera is a disulfide-linked homodimeric protein. The reduced monomer consists of 562 amino acids and predictes a molecular mass of 61.1 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhAGER/Fc monomer is approximately 80-90 kDa due to glycosylation. |
| predicted N | Gln 24 |
| SDS-PAGE | 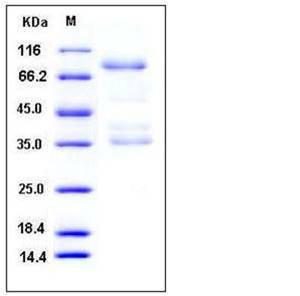 |
| Purity | > 70 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human AGER isoform 1 (NP_001127.1) extracellular domain (Met 1-Ala 344) was fused with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | Measured by its binding ability in a functional ELISA. Immobilized human S100A12 at 2 μg/ml (100 μl/well) can bind recombinant human AGER with a linear range of 0.032-20 μg/ml. |
| Research Area | Cardiovascular |Atherosclerosis |Vascular Inflammation |Inflammatory mediators |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Receptor for Advanced Glycosylation End Products (RAGE, or AGER) is a member of the immunoglobulin super-family transmembrane proteins, as a signal transduction receptor which binds advanced glycation endproducts, certain members of the S100/calgranulin family of proteins, high mobility group box 1 (HMGB1), advanced oxidation protein products, and amyloid (beta-sheet fibrils). Initial studies investigating the role of RAGE in renal dysfunction focused on diabetes, neurodegenerative disorders, and inflammatory responses. However, RAGE also has roles in the pathogenesis of renal disorders that are not associated with diabetes, such as obesity-related glomerulopathy, doxorubicin-induced nephropathy, hypertensive nephropathy, lupus nephritis, renal amyloidosis, and ischemic renal injuries. RAGE represents an important factor in innate immunity against pathogens, but it also interacts with endogenous ligands, resulting in chronic inflammation. RAGE signaling has been implicated in multiple human illnesses, including atherosclerosis, arthritis, Alzheimer's disease, atherosclerosis and aging associated diseases. |
| Reference |
