Human AGO2 / Argonaute 2 / EIF2C2 Protein (His Tag)
Argonaute 2,EIF2C2,Q10
- 100ug (NPP3567) Please inquiry
| Catalog Number | P11079-H07B |
|---|---|
| Organism Species | Human |
| Host | Baculovirus-Insect Cells |
| Synonyms | Argonaute 2,EIF2C2,Q10 |
| Molecular Weight | The recombinant human AGO2 consists of 877 amino acids and predicts a molecular mass of 99 kDa as estimated by SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 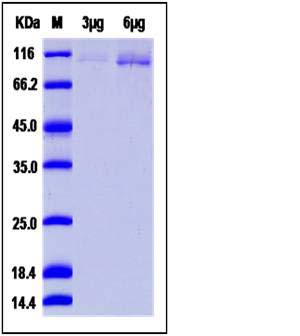 |
| Purity | > 85 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the full length of human AGO2 (NP_036286.2) (Met 1-Ala 859) was expressed with a polyhistidine tag at the N-terminus. |
| Bio-activity | Human AGO2 (11079-H07B) can bind Let-7a RNA and cleave target RNA (21nt). |
| Research Area | Epigenetics |RNAi |Argonaute and Piwi |
| Formulation | Lyophilized from sterile 20mM Tris, 500mM NaCl, pH7.4,10% glycerol,2mM DTT 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Argonaute 2 (AGO2), also known as Eukaryotic translation initiation factor 2C2 (EIF2C2), belongs to the Argonaute family, AGO subfamily, which is a component of the RNA-induced silencing complex (RISC) and mediates small interfering RNA (siRNA)-directed mRNA cleavage and microRNA translational suppression. AGO2 protein is the catalytic engine of mammalian RNAi. It contains a PIWI domain that is structurally related to RNases H and possibly shares with them a two-metal-ion catalysis mechanism. Human AGO2 was unable to cleave preformed RNA duplexes and exhibited weaker binding affinity for RNA duplexes compared with the single strand RNA. The enzyme exhibited greater RNase H activity in the presence of Mn2+ compared with Mg2+. Human AGO2 exhibited weaker binding affinities and reduced cleavage activities for antisense RNAs with either a 5'-terminal hydroxyl or abasic nucleotide. In mouse hematopoiesis, AGO2 controls early development of lymphoid and erythroid cells. AGO2 is a highly specialized member of the Argonaute family with an essential nonredundant Slicer-independent function within the mammalian miRNA pathway. AGO2 regulates dFMR1 expression, and the relationship between dFMR1 and AGO2 was defined by their physical interaction and co-regulation of downstream targets. AGO2 and dFMR1 are also connected through a regulatory relationship. AGO2 is a regulator of dFMR1 expression and have clarified an important developmental role for AGO2 in the nervous system and germ line that requires dFMR1 function. In addition, AGO2 is regulated at both the transcriptional and posttranslational level, and also implicate AGO2 and enhanced micro-RNA activity in the tumorigenic progression of breast cancer cell lines. |
| Reference |
