Human AKR1B1 Protein (His Tag)
ADR,ALDR1,ALR2,AR,MGC1804
- 100ug (NPP1882) Please inquiry
| Catalog Number | P11294-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | ADR,ALDR1,ALR2,AR,MGC1804 |
| Molecular Weight | The recombinant human AKR1B1 comprises 332 amino acids and has a predicted molecular mass of 37.9 kDa. It migrates as an approximately 36 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 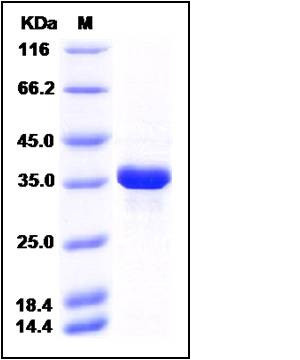 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human AKR1B1 (P15121) (Met 1-Phe 316) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile PBS, 20% glycerol, pH 7.5 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Aldose reductase (AKR1B1) belongs to the aldo/keto reductase superfamily. AKR1B1 is a NADPH-dependent aldo-keto reductase best known as the rate-limiting enzyme of the polyol pathway. Expression of AKR1B1 was the highest in lens and retina. It is the first enzyme in the polyol pathway through which glucose is converted to sorbitol which is important for the function of various organs in the body, and has been implicated in the etiology of diabetic complications. AKR1B1 is quite abundant in the collecting tubule cells and thought to provide protection against hypertonic environment. Some human tissues contain AKR1B1 as well as AKR1B10, a closely related member of the aldo-keto reductase superfamily. |
| Reference |
