Human APP / Protease nexin-II Protein (Fc Tag)
AAA,ABETA,ABPP,AD1,APPI,CTFgamma,CVAP,PN-II,PN2
- 100ug (NPP3604) Please inquiry
| Catalog Number | P10703-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | AAA,ABETA,ABPP,AD1,APPI,CTFgamma,CVAP,PN-II,PN2 |
| Molecular Weight | The recombinant human APP/Fc is a disulfide-linked homodimeric protein after removal of the signal peptide. The reduced monomer consists of 890 amino acids and predicts a molecular mass of 101 kDa. By SDS-PAGE under reducing conditions, the apparent molecular mass of rhAPP/Fc monomer is approximately 150-160 kDa due to the glycosylation. |
| predicted N | Leu 18 |
| SDS-PAGE | 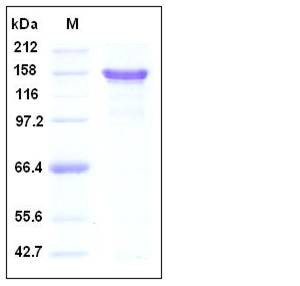 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human APP-751 isoform (NP_958816.1) (Met 1-Leu 669) was expressed with the C-terminal fused Fc region of human IgG1. |
| Bio-activity | Measured by its ability to inhibit trypsin cleavage of a fluorogenic peptide substrate, Mca-RPKPVE-Nval-WRK(Dnp)-NH2, (R&D Systems, Catalog # ES002). The IC50 value is < 1.2 nM. |
| Research Area | Cancer |Signal transduction |Other Signal Transduction Molecules |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Amyloid precursor protein (APP) is a type I transmembrane protein expressed in many tissues and concentrated in the synapses of neurons, and is suggested as a regulator of synapse formation and neural plasticity. APP can be processed by two different proteolytic pathways. In one pathway, APP is cleaved by β- and γ-secretase to produce the amyloid-β-protein (Aβ, Abeta, beta-amyloid) which is the principal component of the amyloid plaques, the major pathological hallmark of Alzheimer’s disease (AD), while in the other pathway, α-secretase is involved in the cleavage of APP whose product exerts antiamyloidogenic effect and prevention of the Aβ peptide formation. The aberrant accumulation of aggregated beta-amyloid peptides (Abeta) as plaques is a hallmark of AD neuropathology and reduction of Abeta has become a leading direction of emerging experimental therapies for the disease. Besides this pathological function of Abeta, recently published data reveal that Abeta also has an essential physiological role in lipid homeostasis. Cholesterol increases Abeta production, and conversely A beta production causes a decrease in cholesterol synthesis. Abeta may be part of a mechanism controlling synaptic activity, acting as a positive regulator presynaptically and a negative regulator postsynaptically. The pathological accumulation of oligomeric Abeta assemblies depresses excitatory transmission at the synaptic level, but also triggers aberrant patterns of neuronal circuit activity and epileptiform discharges at the network level. Abeta-induced dysfunction of inhibitory interneurons likely increases synchrony among excitatory principal cells and contributes to the destabilization of neuronal networks. There is evidence that beta-amyloid can impair blood vessel function. Vascular beta-amyloid deposition, also known as cerebral amyloid angiopathy, is associated with vascular dysfunction in animal and human studies. Alzheimer disease is associated with morphological changes in capillary networks, and soluble beta-amyloid produces abnormal vascular responses to physiological and pharmacological stimuli. |
| Reference |
