Human ASGPR1 / ASGR1 Protein (His Tag)
ASGPR,ASGPR1,CLEC4H1,HL-1
- 100ug (NPP1913) Please inquiry
| Catalog Number | P10773-H07H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | ASGPR,ASGPR1,CLEC4H1,HL-1 |
| Molecular Weight | The recombinant human ASGR1 consists of 246 amino acids and has a calculated molecular mass of 28.8 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhASGR1 is approximately 37 kDa due to glycosylation. |
| predicted N | His |
| SDS-PAGE | 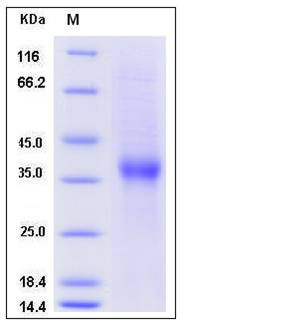 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human ASGR1 isoform 1 (P07306) (Gln 62-Leu 291) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Microbiology |Pathogenic microorganism |viruses |animal virus |RNA virus |+ssRNA virus | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | The asialoglycoprotein receptor (ASGPR), an endocytotic cell surface receptor expressed by hepatocytes, is triggered by triantennary binding to galactose residues of macromolecules such as asialoorosomucoid (ASOR). ASGPR belongs to the long-form subfamily of the C-type/Ca2+ dependent lectin family. It is a complex of two noncovalently-linked and highly homologous subunits, a major 42 kDa glycoprotein ASGPR1(MHL-1) and a minor 51 kDa glycoprotein ASGR2 (MHL-2). ASGPR1 is synthesized as a type II transmembrane protein that contains a cytosolic N-terminal domain, a single transmembrane segment, and an extracellular domain which contains two important structural regions. The first is a stalk domain that contributes to noncovalent oligomerization, and the second is a Ca2+-dependent carbohydrate binding domain at the very C-terminus that is unusually stabilized by three ions. The research regarded that ASGPR1 could be targeted for anti- hepatitis B virus (HBV) drug development. |
| Reference |
