Human ATG8 / GABARAPL1 Protein (His Tag)
APG8-LIKE,APG8L,ATG8,ATG8B,ATG8L,GEC1
- 100ug (NPP1915) Please inquiry
| Catalog Number | P14549-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | APG8-LIKE,APG8L,ATG8,ATG8B,ATG8L,GEC1 |
| Molecular Weight | The recombinant human GABARAPL1 consists of 132 amino acids and predicts a molecular mass of 15.9 KDa. It migrates as an approximately 20-22 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | His |
| SDS-PAGE | 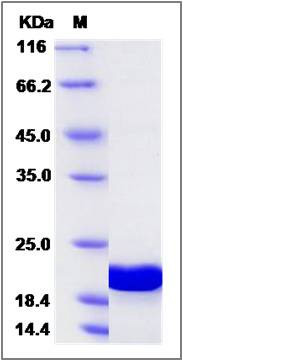 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human GABARAPL1 (Q9H0R8) (Met1-Lys117) was expressed with a polyhistide tag at the N-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Neurotransmitter Receptors, Transporters, and Ion Channels |GABA Receptors |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | ATG8, also known as GABARAPL1, is a ubiquitin-like protein which has a crystal structure. ATG8 consists of a 5-stranded β-sheet, which is enclosed by two α-helices at one side and one α-helix at the other side and exhibits a conserved GABARAP domain. It functions in the formation of autophagosomal membranes. The transient conjugation of ATG8 to the autophagosomal membrane through a ubiquitin-like conjugation system is essential for autophagy in eukaryotes. Autophagy is induced upon nutrient depletion or rapamycin treatment and leads to the response of more than 30 autophagy-related (ATG) genes known so far, including ATG8. |
| Reference |
