Human BACE1 / ASP2 Protein (Fc Tag)
ASP2,BACE,HSPC104
- 100ug (NPP3592) Please inquiry
| Catalog Number | P10064-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | ASP2,BACE,HSPC104 |
| Molecular Weight | The recombinant human BACE1/Fc is a disulfide-linked homodimeric protein. The reduced monomer consists of 674 amino acids and has a calculated molecular mass of 75 kDa. As a result of glycosylation, the rhBACE1/Fc monomer migrates as an approximately 100-105 kDa protein in SDS-PAGE under reducing conditions. |
| predicted N | Thr 22 |
| SDS-PAGE | 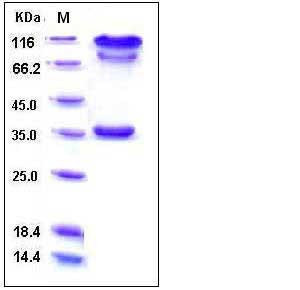 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Thr 457) of human BACE1 (NP_036236.1) was expressed as a secreted chimeric protein with the C-terminal fused Fc region of human IgG1. |
| Bio-activity | Measured by its ability to cleave a fluorogenic peptide substrate, Mca-SEVNLDAEFRK(Dpn)RR-NH2, (R&D Systems, Catalog # ES004). The specific activity is >0.5 pmoles/min/μg. |
| Research Area | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Beta-site APP-cleaving enzyme 1 (BACE1) is an aspartic-acid protease important in the formation of myelin sheaths in peripheral nerve cells. In the brain, This protein is expressed highly in the substantia nigra, locus coruleus and medulla oblongata. Strong BACE1 expression has also been described in pancreatic tissue. BACE1 has a pivotal role in the pathogenesis of Alzheimer's disease. In Alzheimer's disease patients, BACE1 levels were elevated although mRNA levels were not changed. It has been found that BACE1 gene expression is controlled by a TATA-less promoter. The translational repression as a new mechanism controlling its expression. And the low concentrations of Ca(2+) (microM range) significantly increased the proteolytic activity of BACE1. Furthermore, BACE1 protein is ubiquitinated, and the degradation of BACE1 proteins and amyloid precursor protein processing are regulated by the ubiquitin-proteasome pathway. It has also been identified as the rate limiting enzyme for amyloid-beta-peptide (Abeta) production. |
| Reference |
