Human C2 / Complement Component 2 Protein (His Tag)
ARMD14,CO2
- 100ug (NPP3757) Please inquiry
| Catalog Number | P10154-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | ARMD14,CO2 |
| Molecular Weight | The single-chain form of recombinant human complement component C2 comprises 743 amino acids and has a calculated molecular mass of 82.5 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhC2 is 90-100 kDa as a result of glycosylation. |
| predicted N | Ala 21 |
| SDS-PAGE | 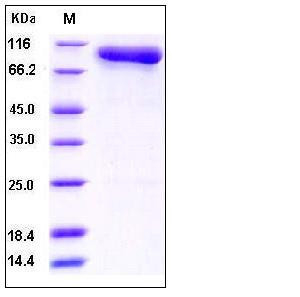 |
| Purity | > 97 % as determined by SDS-PAGE and SEC-HPLC Analysis. |
| Protein Construction | A DNA sequence encoding the human complement component 2 (C2) precursor (NP_000054.2) (Met 1-Leu 752) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to cleave a colorimetric peptide substrate, N-carbobenzyloxy-Gly-Arg-ThioBenzyl ester (Z-GR-SBzl), in the presence of 5,5’Dithiobis (2-nitrobenzoic acid) (DTNB). The specific activity is >100 pmoles/min/μg. |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Plasma Cascade Systems in Inflammation |Complement System |Classical Pathway |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Complement component C2 is part of the classical complement pathway which plays a major role in innate immunity against infection. C2 is a glycoprotein synthesized in liver hepatocytes and several other cell types in extrahepatic tissues. This pathway is triggered by a multimolecular complex C1, and subsequently the single-chain form of C2 is cleaved into two chains referred to C2a and C2b by activated C1. The second component of complement (C2) is a multi-domain serine protease that provides catalytic activity for the C3 and C5 convertases of the classical and lectin pathways of human complement. C4b and C2 was investigated by surface plasmon resonance. C2a containing a serine protease domain combines with complement component C4b to form the C3 convertase C4b2a which is responsible for C3 activation, and leads to the stimulation of adaptive immune responses via Lectin pathway. C2 bound to C4b is cleaved by classical (C1s) or lectin (MASP2) proteases to produce C4bC2a. C2 has the same serine protease domain as C4bC2a but in an inactive zymogen-like conformation, requiring cofactor-induced conformational change for activity. Deficiency of C2 (C2D) is the most common genetic deficiency of the complement system, and two types of C2D have been recognized in the context of specific MHC haplotypes. C2D in human is reported to increase susceptibility to infection, and is associated with certain autoimmune diseases, such as rheumatological disorders. |
| Reference |
