Human CALR / Calreticulin Protein (Fc Tag)
cC1qR,CRT,HEL-S-99n,RO,SSA
- 100ug (NPP3629) Please inquiry
| Catalog Number | P13539-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | cC1qR,CRT,HEL-S-99n,RO,SSA |
| Molecular Weight | The recombinant human CALR/Fc is a disulfide-linked homodimer. The reduced monomer comprises 637 amino acids and has a predicted molecular mass of 73 kDa. The apparent molecular mass of the protein is approximately 96 and 38 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Glu 18 |
| SDS-PAGE | 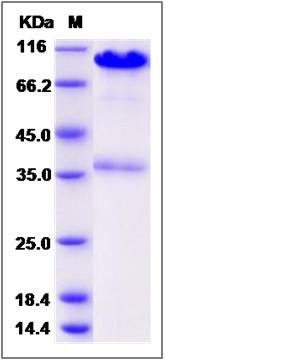 |
| Purity | (75.9+20.5) % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human CALR (P27797) (Met1-Ala413) was expressed with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | |
| Research Area | Cancer |Signal transduction |Neurotransmitter Receptors, Transporters, and Ion Channels |Calcium-binding Proteins and Related Molecules |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Calreticulin is a multifunctional protein. It acts as a main Ca(2+)-binding (storage) protein in the lumen of the endoplasmic reticulum. Calreticulin binds Ca2+ ions (a second messenger in signal transduction), rendering it inactive. The Ca2+ is bound with low affinity, but high capacity, and can be released on a signal. Located in storage compartments associated with the endoplasmic reticulum, calreticulin also binds to misfolded proteins and prevents them from being exported from the endoplasmic reticulum to the golgi apparatus. The amino terminus of calreticulin interacts with the DNA-binding domain of the glucocorticoid receptor and prevents the receptor from binding to its specific glucocorticoid response element. Calreticulin reduces the binding of androgen receptor to its hormone-responsive DNA element and inhibits androgen receptor and retinoic acid receptor transcriptional activities in vivo, as well as retinoic acid-induced neuronal differentiation. Therefore, calreticulin acts as a significant modulator of the regulation of gene transcription by nuclear hormone receptors. |
| Reference |
