Human CANT1 Protein (Fc Tag)
DBQD,SCAN-1,SCAN1,SHAPY
- 100ug (NPP1956) Please inquiry
| Catalog Number | P13124-H01H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | DBQD,SCAN-1,SCAN1,SHAPY |
| Molecular Weight | The recombinant human CANT1/Fc is a disulfide-linked homodimer. The reduced monomer comprises 582 amino acids and has a predicted molecular mass of 64.3 kDa. The apparent molecular mass of the protein is approximately 65 kDa in SDS-PAGE under reducing conditions due to glycosylation. |
| predicted N | Glu |
| SDS-PAGE | 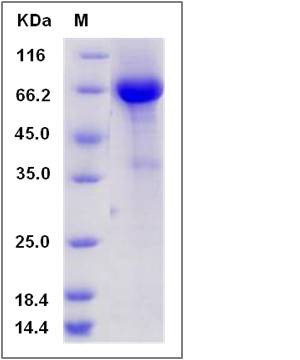 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human CANT1 (Q8WVQ1-1) (Gly80-Ile401) was expressed,with the fused Fc region of human IgG1 at the N-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Protein Trafficking |Golgi Proteins |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | CANT1(calcium activated nucleotidase 1) belongs to the apyrase family. Apyrase is a calcium-activated plasma membrane-bound enzyme (magnesium can also activate it) (EC 3.6.1.5) that catalyses the hydrolysis of ATP to yield AMP and inorganic phosphate. Two isoenzymes are found in commercial preparations from S. tuberosum. One with a higher ratio of substrate selectivity for ATP: ADP and another with no selectivity. It can also act on ADP and other nucleoside triphosphates and diphosphates with the general reaction being NTP -> NDP + Pi -> NMP + 2Pi. The salivary apyrases of blood-feeding arthropods are nucleotide hydrolysing enzymes are implicated in the inhibition of host platelet aggregation through the hydrolysis of extracellular adenosine diphosphate. CANT1 functions as a calcium-dependent nucleotidase with a preference for UDP. Defects in CANT1 are the cause of desbuquois dysplasia. |
| Reference |
