Human CD112 / Nectin-2 / PVRL2 Protein (His Tag)
CD112,HVEB,Nectin-2,PRR2,PVRR2
- 100ug (NPP1110) Please inquiry
| Catalog Number | P10005-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CD112,HVEB,Nectin-2,PRR2,PVRR2 |
| Molecular Weight | The recombinant mature human CD112 consists 340 amino acids and has a calculated molecular mass of 36.2 kDa. As a result of glycosylation, the recombinant protein migrates as an approximately 48 kDa protein in SDS-PAGE under reducing conditions. |
| predicted N | Gln 32 |
| SDS-PAGE | 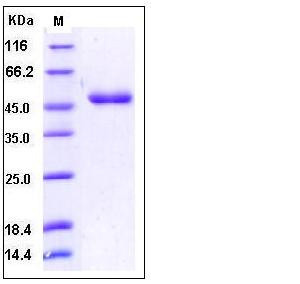 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Leu 360) of human CD112 (NP_002847.1) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its binding ability in a functional ELISA . Immobilized recombinant human CD112 at 20 μg/ml (100 μl/well) can bind biotinylated DNAM1 with a linear range of 0.078-2.5 μg/ml . |
| Research Area | Developmental Biology |Embryogenesis |Germ Layer Formation |Ectoderm Marker |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Cluster of Differentiation 112 (CD112), also known as poliovirus receptor related protein 2 (PVRL2 or PRR2), is a single-pass type I transmembrane glycoprotein belonging to the Immunoglobulin superfamily. CD112 protein also serves as an entry for certain mutant strains of herpes simplex virus and pseudorabies virus, and thus is involved in cell to cell spreading of these viruses. CD112 protein has been identified as the ligand for DNAM-1 (CD226), and the interaction of CD226/CD112 protein can induce NK cell- and CD8+ T cell-mediated cytotoxicity and cytokine secretion. CD112 has been regarded as a critical component in allergic reactions, and accordingly may function as a novel target for anti-allergic therapy. |
| Reference |
