Human CD89 / FCAR Protein (His Tag)
CD89,CTB-61M7.2,FcalphaRI,FCAR,XXbac-BPG230H20.5
- 100ug (NPP3718) Please inquiry
| Catalog Number | P10414-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CD89,CTB-61M7.2,FcalphaRI,FCAR,XXbac-BPG230H20.5 |
| Molecular Weight | The secreted recombinant human CD89 is a monomeric protein consisting of 217 amino acids and predicts a molecular mass of 25 kDa. The apparent molecular mass of rhCD89 is approximately 45-50 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Gln 22 |
| SDS-PAGE | 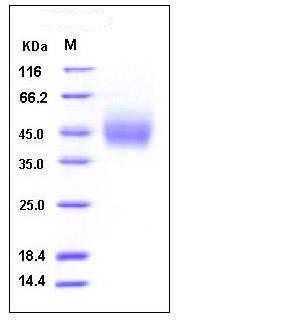 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the pro form of human FCAR extracellular domain (NP_001991.1) (Met 1-Asn 227) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to bind human IgA in functional Elisa. |
| Research Area | Immunology |Signal Transduction |ITIM/ITAM Immunoreceptors and Related Molecules |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | FCAR, also called FcαRI or CD89, is a type I transmembrane receptor for Fc region of IgA which is the most abundant immunoglobulin in mucosal areas but is only the second most common antibody isotype in serum. This receptor is present on the surface of myeloid lineage cells such as neutrophils, monocytes, macrophages, and eosinophils, especially phagocytes located in mucosal areas. Upon ligand IgA binding, FcαRI associates with the FcR γ signaling molecule bearing the immunoreceptor tyrosine-based activation motif (ITAM) through a unique charge-based mechanism and triggers multiple cell-mediated immune responses. It has been reported that Fc RI is a dual-function receptor that can mediate both inflammatory and anti-inflammatory responses depending on the type of interaction with its ligand. Sustained aggregation of FCAR results in activation of target-cell functions such as antigen presentation and cytokine release. In contrast, Monomeric targeting with serum IgA or with a variety of anti-FcαRI Fab fragments triggers an inhibitory response and additionally induces apoptosis. FcαRI thus play an fundamental role in preventing tumor development and growth, as well as in controlling inflammation. |
| Reference |
|
