Human CD93 / C1QR1 Protein (His Tag)
C1QR1, C1qR(P), C1qRP, CDw93, ECSM3, MXRA4, dJ737E23.1
- 100ug (NPP3721) Please inquiry
| Catalog Number | P12589-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | C1QR1, C1qR(P), C1qRP, CDw93, ECSM3, MXRA4, dJ737E23.1 |
| Molecular Weight | The secreted recombinant human CD93 comprises 570 amino acids and has a predicted molecular mass of 59.6 kDa. As a result of glycosylation, rh CD93 migrates as an approximately 100 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Thr 22 |
| SDS-PAGE | 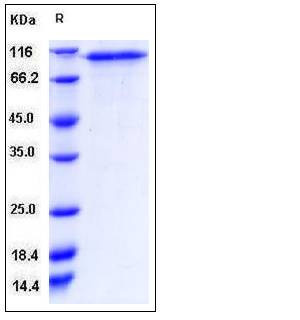 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human CD93 (Q9NPY3) extracellular domain (Met 1-Lys 580) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Immunology |Innate Immunity |Complement System |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | CD93 or C1q receptor 1 (C1qR) is an about 120 kDa O-sialoglycoprotein that within the hematopoietic system is selectively expressed on cells of the myeloid lineage. CD93/C1qR is a highly glycosylated transmembrane protein expressed on monocytes, neutrophils, endothelial cells, and stem cells. CD93 was originally identified as a myeloid cell-surface marker and subsequently associated with an ability to modulate phagocytosis of suboptimally opsonized immunoglobulin G and complement particles in vitro. CD93/C1qR, a receptor expressed during early B-cell development, is reinduced during plasma-cell differentiation. High CD93/CD138 expression was restricted to antibody-secreting cells both in T-dependent and T-independent responses as naive, memory, and germinal-center B cells remained CD93-negative. CD93 was expressed on (pre)plasmablasts/plasma cells, including long-lived plasma cells that showed decreased cell cycle activity, high levels of isotype-switched Ig secretion, and modification of the transcriptional network. CD93 is important for the maintenance of plasma cells in bone marrow niches. |
| Reference |
