Human CFL2 / cofilin 2 / ADF Protein (His Tag)
NEM7
- 100ug (NPP2008) Please inquiry
| Catalog Number | P11463-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | NEM7 |
| Molecular Weight | The recombinant human CFL2 consisting of 180 amino acids and has a calculated molecular mass of 20.4 kDa. The apparent molecular mass of the protein is approximately 21 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 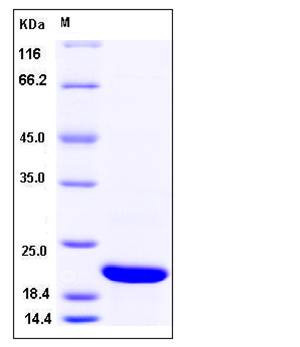 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human CFL2 (Q9Y281-1) (Ala 2-Leu 166) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Neuroscience |Cell Adhesion Proteins |Cytoskeletal Proteins |Microfilaments |Actin etc |Actin Binding Proteins | |
| Formulation | Lyophilized from sterile PBS, pH 7.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Cofilin 2 (muscle), also known as CFL2, is a member of cofilin family of the actin-binding protein superfamily. Cofilin2 shows significant homology to the other two members: cofilin 1 and DSTN, through its entire sequence, and contains residues conserved among the cofilin family that are responsible for actin-binding. Cofilin 2 (CFL2) is an important regulator of striated myocyte function. Purified cofilin 2 depolymerized actin filaments in a dose- and pH-dependent manner and reduced the apparent viscosity of an actin solution, although they did not co-sediment with actin filaments at all. Cofilin2 is not expressed in vegetative cells, but is transiently induced during the aggregation stage of development, whereas cofilin 1 was predominantly expressed in vegetative cells. |
| Reference |
