Human CLEC4A / CLECSF6 / DCIR Protein (His Tag)
CD367,CLEC4A,CLECSF6,DCIR,DDB27,HDCGC13P,LLIR
- 100ug (NPP3795) Please inquiry
| Catalog Number | P11476-H07H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CD367,CLEC4A,CLECSF6,DCIR,DDB27,HDCGC13P,LLIR |
| Molecular Weight | The recombinant human CLEC4A consists of 184 amino acids and has a calculated molecular mass of 22 kDa. In SDS-PAGE under reducing conditions, rhCLEC4A migrates as an approximately 28 kDa band due to glycosylation. |
| predicted N | His |
| SDS-PAGE | 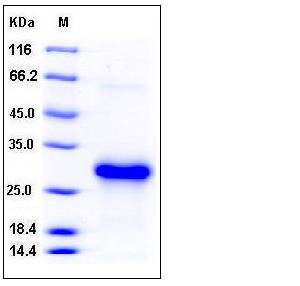 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human CLEC4A (NP_057268.1) extracellular domain (Gln 70-Leu 237) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Pattern Recognition Receptors |C-type Lectin Receptors |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Dendritic cell immunoreceptor (DCIR), also known as C-type lectin domain family 4 member A (CLEC4A), C-type lectin superfamily member 6 (CLECSF6), is a single-pass type II C-type lectin receptor expressed mainly in dendritic cells (DCs), which is a negative regulator of DC expansion and has a crucial role in maintaining the homeostasis of the immune system. The Dectin-2 family of C-type lectins that includes Dectin-2, BDCA-2, DCIR, DCAR, Clecsf8 and Mincle. These type II receptors contain a single extracellular carbohydrate recognition domain and have diverse functions in both immunity and homeostasis. DCIR is the only member of the family which contains a cytoplasmic signalling motif and has been shown to act as an inhibitory receptor, while BDCA-2, Dectin-2, DCAR and Mincle all associate with FcRgamma chain to induce cellular activation, including phagocytosis and cytokine production. Dectin-2 and Mincle have been shown to act as pattern recognition receptors for fungi, while DCIR acts as an attachment factor for HIV. In addition to pathogen recognition, DCIR has been shown to be pivotal in preventing autoimmune disease by controlling dendritic cell proliferation. DCIR expressed on antigen presenting cells and granulocytes and acts as an inhibitory receptor via an intracellular immunoreceptor tyrosine-based inhibitory motif (ITIM). It may also be involved via its ITIM motif in the inhibition of B-cell-receptor-mediated calcium mobilization and protein tyrosine phosphorylation. Additionally, DCIR can participate in the capture of HIV-1 and promote infection in trans and in cis of autologous CD4(+) T cells from human immature monocyte-derived DCs. DCIR acts as a ligand for HIV-1 and is involved in events leading to productive virus infection. |
| Reference |
