Human CMBL Protein (His Tag)
JS-1
- 100ug (NPP3746) Please inquiry
| Catalog Number | P11404-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | JS-1 |
| Molecular Weight | The recombinant human CMBL comprises 260 amino acids and has a calculated molecular mass of 30 kDa. It migrates as an approximately 28 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 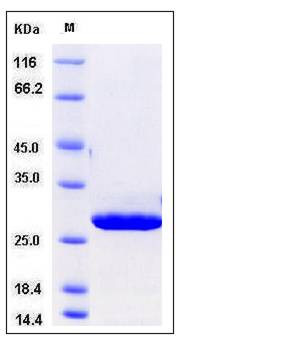 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human CMBL (Q96DG6) (Met 1-Met 245) was expressed, with a N-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile 20mM Tris, 0.1% Brij35, pH 8.0 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Carboxymethylenebutenolidase (CMBL), also known as 4-carboxymethylenebut-2-en-4-olide lactonohydrolase, maleylacetate enol- lactonase, dienelactone hydrolase, and carboxymethylene butenolide hydrolase, is a hydrolase specially belonging to the family of hydrolases. It maily acts on carboxylic ester bonds. CMBL is a human homolog of Pseudomonas dienelactone hydrolase involved in the bacterial halocatechol degradation pathway. The ubiquitous expression of human CMBL gene transcript in various tissues was observed. CMBL was demonstrated to be the primary olmesartan medoxomil (OM) bioactivating enzyme in the liver and intestine. The recombinant human CMBL expressed in mammalian cells was clearly shown to activate OM. The recombinant CMBL also converted other prodrugs having the same ester structure as OM, faropenem medoxomil and lenampicillin, to their active metabolites. CMBL exhibited a unique sensitivity to chemical inhibitors, thus, being distinguishable from other known esterases. |
| Reference |
