Human CPLX3 / Complexin 3 Protein (His Tag)
CPX-III,CPXIII,Nbla11589
- 100ug (NPP2046) Please inquiry
| Catalog Number | P14518-H07H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CPX-III,CPXIII,Nbla11589 |
| Molecular Weight | The recombinant human CPLX3 comprises 174 amino acids and has a predicted molecular mass of 19.5 kDa. The apparent molecular mass of the protein is approximately 29-32 kDa in SDS-PAGE under reducing conditions. |
| predicted N | His |
| SDS-PAGE | 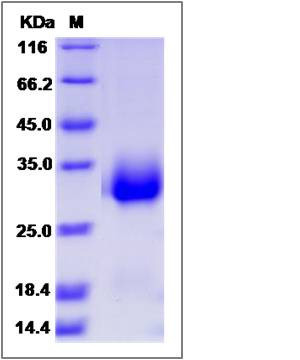 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human CPLX3 (Q8WVH0) (Met1-Lys154) was expressed with an N-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | Neuroscience |Neurotransmission |Secretory Vesicles |Regulation |
| Formulation | Lyophilized from sterile PBS, pH 7.4. 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | CPLX3, also known as complexin 3, belongs to the complexin/synaphin family. As a SNARE-binding protein, complexin (CPX), can act either as a facilitator or as an inhibitor of membrane fusion, constituting a controversial dilemma. CPX acts sequentially on assembling SNAREpins, first facilitating zippering by nearly doubling the distance at which v- and t-SNAREs can engage and then clamping them into a half-zippered fusion-incompetent state. Specifically, the central helix of CPX allows SNAREs to form this intermediate energetic state at 9-15 nm but not when the bilayers are closer than 9 nm. Stabilizing the activated-clamped state at separations of less than 9 nm requires the accessory helix of CPX, which prevents membrane-proximal assembly of SNAREpins. CPLX3 binds to the SNARE core complex containing SNAP25, VAMP2 and STX1A. |
| Reference |
