Human CTRC / chymotrypsin C Protein (His Tag)
CLCR,ELA4
- 100ug (NPP3784) Please inquiry
| Catalog Number | P11456-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CLCR,ELA4 |
| Molecular Weight | The secreted recombinant human CTRC (pro form) consists of 263 amino acids and has a predicted molecular mass of 29.3 kDa. It migrates as an approximately 36 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Cys 17 |
| SDS-PAGE | 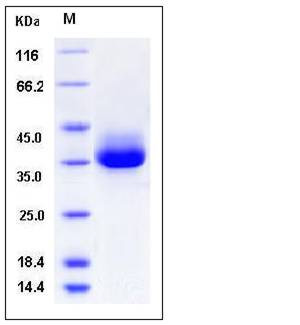 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human CTRC (Q99895) (Met 1-Leu 268) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Developmental Biology |Metabolism |Types of disease |Metabolism in Cancer |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Chymotrypsin C (abbreviated for CTRC), also known as caldecrin or elastase4, is a digestive enzyme of the peptidase S1 family. This enzyme is synthesized as an inactivate chymotrypsinogen. On cleavage by trypsin into two parts that activate each other by removing two small peptides in a trans-proteolysis, chymotrypsin C produced. N-linked glycosylation of human CTRC is required for efficient folding and secretion, however, the N-linked glycan is unimportant for enzyme activity or inhibitor binding. It has been proposed that CTRC is a key regulator of digestive zymogen activation and a physiological co-activator of digestive carboxypeptidases proCPA1 and proCPA2. Mutations that abolish activity or secretion of CTRC increase the risk for chronic pancreatitis. It's speculated that CTRC might regulate pancreatic cancer cell migration in relation to cytokeratin 18 expression. The pancreatic cancer cell migration ability was downregulated in pancreatic cancer Aspc-1 cells that overexpressed CTRC, whereas the cell migration ability was upregulated in Aspc-1 cells in which CTRC was suppressed. |
| Reference |
