Human Calsequestrin-1 / CASQ1 Protein
CASQ1, CASQ
- 100ug (NPP3641) Please inquiry
| Catalog Number | P13805-HNAE |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | CASQ1, CASQ |
| Molecular Weight | The recombinant human CASQ1 consists of 363 amino acids and predicts a molecular mass of 41.8 KDa. It migrates as an approximately 52 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 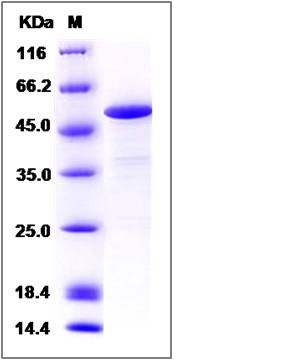 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mature form of human CASQ1 (P31415) (Gln35-Asp396) was expressed with a N-terminal Met. |
| Bio-activity | |
| Research Area | Neuroscience |Cell Type Marker in Neurodevelopment |Neuronal Cell Markers |Synapse & Synaptic Proteins |Calcium Signaling |Calcium Binding Proteins | |
| Formulation | Lyophilized from sterile 50mM Tris, 10% glycerol, pH 7.5. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Calsequestrin-1 is an isoform of calsequestrin. Calsequestrin is a calcium-binding protein of the sarcoplasmic reticulum. It helps hold calcium in the cisterna of the sarcoplasmic reticulum after a muscle contraction, even though the concentration of calcium in the sarcoplasmic reticulum is much higher than in the cytosol. Two forms of calsequestrin have been identified: Calsequestrin-2 and Calsequestrin-1. Calsequestrin-1 is found in fast skeletal muscle. The release of calsequestrin-bound calcium (through a calcium release channel) triggers muscle contraction. The active protein is not highly structured, more than 50% of it adopting a random coil conformation. When calcium binds there is a structural change whereby the alpha-helical content of the protein increases from 3 to 11%. Both forms of calsequestrin are phosphorylated by casein kinase 2, but the cardiac form is phosphorylated more rapidly and to a higher degree. Calsequestrin-1 is also secreted in the gut where it deprives bacteria of calcium ions. |
| Reference |
