Human Carbonic Anhydrase II / CA2 Protein (His Tag)
CA-II,CAC,CAII,Car2,HEL-76,HEL-S-282
- 100ug (NPP1959) Please inquiry
| Catalog Number | P10478-H08E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | CA-II,CAC,CAII,Car2,HEL-76,HEL-S-282 |
| Molecular Weight | The recombinant human CA2 consisting of 266 amino acids and has a calculated molecular mass of 30 kDa as estimated in SDS-PAGE under reducing conditions. |
| predicted N | Met 1 |
| SDS-PAGE | 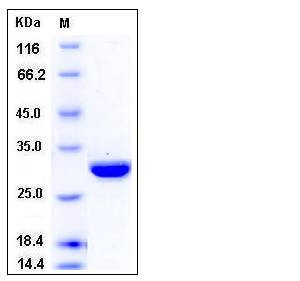 |
| Purity | > 96 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human CA2 (NP_000058.1) (Met 1-Lys 260) was expressed, with a polyhistide tag at the C-terminus. |
| Bio-activity | Measured by its esterase activity. The specific activity is >150 pmoles/min/μg . |
| Research Area | Signaling |Signal Transduction |Metabolism |Pathways and Processes |Metabolism processes |Hypoxia | |
| Formulation | Lyophilized from sterile 20mM Tris 0.5M NaCl, pH 8.0 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | The carbonic anhydrases (or carbonate dehydratases) are classified as metalloenzyme for its zinc ion prosthetic group and form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons, a reversible reaction that takes part in maintaining acid-base balance in blood and other tissues. The carbonic anhydrasekl (CA) family consists of at least 11 enzymatically active members and a few inactive homologous proteins. Carbonic anhydrase II is one of fourteen forms of human α carbonic anhydrases. Defects in this enzyme are associated with osteopetrosis and renal tubular acidosis. Renal carbonic anhydrase allows the reabsorption of sodium ions in the proximal tubule. Carbonic anhydrase II has been shown to interact with Band 3 and Sodium-hydrogen antiporter 1. |
| Reference |
