Human Carbonic Anhydrase III / CA3 Protein (His Tag)
CAIII,Car3
- 100ug (NPP3633) Please inquiry
| Catalog Number | P10503-H08E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | CAIII,Car3 |
| Molecular Weight | The recombinant human CA3 consisting of 266 amino acids and has a calculated molecular mass of 30.4 kDa as estimated in SDS-PAGE under reducing conditions. |
| predicted N | Met 1 |
| SDS-PAGE | 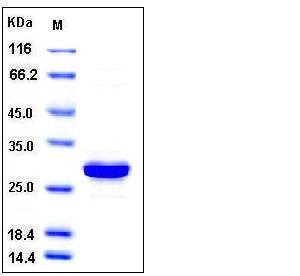 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human CA3 (NP_005172.1) (Met 1-Lys 260) was expressed, with a polyhistide tag at the C-terminus. |
| Bio-activity | Measured by its esterase activity. The specific activity is >5 pmoles/min/μg. |
| Research Area | Cardiovascular |Cardiovascular disease Therapeutic Targets |Hypertension Therapeutic Targets |
| Formulation | Lyophilized from sterile 50mM Tris, 500mM NaCl, 10% glycerol, pH 8.0 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Carbonic anhydrases (CAs) are a large family of zinc metalloenzymes first discovered in 1933 that catalyze the reversible hydration of carbon dioxide. CAs participate in a variety of biological processes, including respiration, calcification, acid-base balance, bone resorption, and the formation of aqueous humor, cerebrospinal fluid, saliva, and gastric acid. Carbonic anhydrases (CAs) form a family of enzymes that catalyze the rapid conversion of carbon dioxide and water to bicarbonate and protons, a reaction that occurs rather slowly in the absence of a catalyst. The active site of most carbonic anhydrases contains a zinc ion, they are therefore classified as metalloenzymes. Several forms of carbonic anhydrase occur in nature. The primary function of the enzyme in animals is to interconvert carbon dioxide and bicarbonate to maintain acid-base balance in blood and other tissues, and to help transport carbon dioxide out of tissues. Plants contain a different form called β-carbonic anhydrase, which, from an evolutionary standpoint, is a distinct enzyme, but participates in the same reaction and also uses a zinc ion in its active site. |
| Reference |
