Human Carboxypeptidase E / CPE Protein (Fc Tag)
CPE
- 100ug (NPP1971) Please inquiry
| Catalog Number | P10069-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CPE |
| Molecular Weight | The recombinant human CPE/Fc is a disulfide-linked homodimeric protein. The reduced monomer consists of 666 amino acids and predicts a molecular mass of 74.6 kDa. As a result of glycosylation, the apparent molecular mass of rhCPE/Fc monomer is approximately 85-90 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Glu 26 |
| SDS-PAGE | 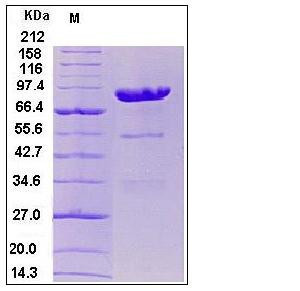 |
| Purity | > 85 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human carboxypeptidase E (CPE) precursor (NP_001864.1) (Met 1-Ser 453) was expressed with the C-terminal fused Fc region of human IgG1. |
| Bio-activity | |
| Research Area | Signaling |Signal Transduction |Cytoskeleton / ECM |Cytoskeletal Proteins |Regulation |
| Formulation | Lyophilized from sterile 100mM Glycine, 10mM NaCl, 50mM Tris, pH 7.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Human carboxypeptidase E (CPE), also known as Carboxypeptidase H, is a peripheral membrane protein and a zinc metallocarboxypeptidase, and the conversion of proCPE into CPE occurs primarily in secretory vesicles. The active form of CPE cleaves C-terminal amino acid residues of the peptide, and is thus involved in the biosynthesis of peptide hormones and neurotransmitters including insulin, enkephalin, etc. The enzymatic activity is enhanced by millimolar concentrations of Co2+. It has also been proposed that membrane-associated carboxypeptidase E acts as a sorting receptor for targeting regulated secretory proteins which are mostly prohormones and neuropeptides in the trans-Golgi network of the pituitary and in secretory granules into the secretory pathway.Its interaction with glycosphingolipid-cholesterol rafts at the TGN facilitates the targeting. Mutations in this gene are implicated in type I I diabetes due to impaired glucose clearance and insulin resistance. |
| Reference |
