Human Cathepsin L1 / CTSL1 Protein (His Tag)
CATL,CTSL1,MEP
- 100ug (NPP3647) Please inquiry
| Catalog Number | P10486-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CATL,CTSL1,MEP |
| Molecular Weight | The recombinant human CTSL1 consists of 327 amino acids and has a predicted molecular mass of 37.3 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of recombinant human CTSL1 is approximately 37 kDa. |
| predicted N | Thr 18 |
| SDS-PAGE | 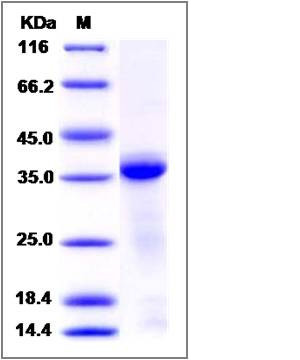 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the pro form of human Cathepsin-L1 (NP_001903.1) (Met 1-Val 333) was expressed, fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | Measured by its binding ability in a functional ELISA . Immobilized human CD74 at 5 μg/ml (100 μl/well) can bind biotinylated human CTSL1 with a linear range of 3.2-400 ng/ml. |
| Research Area | Immunology |Innate Immunity |Lysosomal Enzymes |
| Formulation | Lyophilized from sterile 50 mM NaAc, 100 mM NaCl, pH 7.5. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Cathepsin L is a lysosomal cysteine protease that plays a major role in intracellular protein catabolism, and is potent in degrading collagen, laminin, elastin, as well as alpha-1 protease inhibitor and other structural proteins of basement membranes. It is secreted by liver flukes at all stages of their development in the mammalian host, are believed to play important roles in facilitating parasite migration (tissue degradation), feeding and immuno-evasion. Like many proteases, Cathepsin L is synthesized as an inactive preproenzyme, and cleavage of the 96-residue proregion is necessary to generate the fully active 221-residue mature enzyme. Studies have demonstrated that cleavage of the proregion occur autocatalytically under acidic conditions. The enzyme takes part in nutrient acquisition by catabolizing host proteins to absorbable peptides, facilitates the migration of the parasite through the host intestine and liver by cleaving interstitial matrix proteins such as fibronectin, laminin and native collagen and is implicated in the inactivation of host immune defenses by cleaving immunoglobulins. Recently, Cathepsin L has been shown to suppress Th1 immune response in infected laboratory animals making them susceptible to concurrent bacterial infections. Cathepsin L is synthesized in large amounts and secreted by many malignantly transformed cells, and induced by growth factors and tumor promoters. In addition to its role in protein degradation, evidence has accumulated for the participation of Cathepsin L in various physiological and pathological processes, such as tumor invasion and metastasis, bone resorption, spermatogenesis, and arthritis. Accordingly, Cathepsin L may prove useful as a diagnostic or prognostic marker of human tumor malignancy. |
| Reference |
