Human Coagulation Factor XI / FXI / F11 Protein (His Tag)
FXI
- 100ug (NPP3751) Please inquiry
| Catalog Number | P10302-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | FXI |
| Molecular Weight | The secreted recombinant human F11 consists of 618 amino acids with the predicted molecular mass of 69.5 kDa. As a result of glycosylation, rhCTSS migrates as an approximately 75-80 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Glu 19 |
| SDS-PAGE | 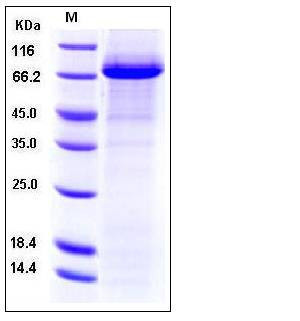 |
| Purity | > 85 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human F11 (NP_000119.1) precursor (Met 1-Val 625) with a carboxy-terminal polyhistidine tag was expressed. |
| Bio-activity | Measured by its ability to cleave the fluorogenic peptide substrate, t-butyloxycarbonyl-Ile-Glu-Gly-Arg-7-amido-4-methylcoumarin (Boc-IEGR-AMC). The specific activity is >100 pmoles/min/μg. (Activation description: The proenzyme needs to be activated by Thermolysin for an activated form) |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Plasma Cascade Systems in Inflammation |Coagulation |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Factor XI (plasma thromboplastin antecedent) is a plasma glycoprotein, and a zymogen acting as a serine protease which participates in blood coagulation as a catalyst in the conversion of factor IX to factor IXa in the presence of calcium ions. It is an unusual dimeric protease, with structural features that distinguish it from vitamin K-dependent coagulation proteases. The factor XI is synthesized in the liver as a single polypeptide chain with a molecular weight estimated between 125 ~160 kDa and then is processed into a disulfide-bond linked homodimer. FXI is a homodimer, with each subunit containing four apple domains and a protease domain. The apple domains form a disk structure with binding sites for platelets, high molecular weight kininogen, and the substrate factor IX (FIX). FXI is converted to the active protease FXIa by cleavage of the Arg369-Ile370 bond on each subunit. After the activation reaction, Factor XIa is composed of two heavy and two light chains held together by three disulfide bonds. The heavy chains are derived from the amino termini of the zymogen and responsible for the binding of factor XI to high molecular weight kininogen and for the activation of factor IX, while the light chain contains the catalytic portion of the enzyme and is homologous to the trypsin family of serine proteases. FXI deficiency is a disorder characterized by a mild or no bleeding tendency. Severe FXI deficiency is an injury-related bleeding disorder common in Ashkenazi Jews and rare worldwide. |
| Reference |
