Human DAPK3 / ZIPK Protein (GST Tag)
DLK,ZIP,ZIPK
- 100ug (NPP3793) Please inquiry
| Catalog Number | P10757-H09B |
|---|---|
| Organism Species | Human |
| Host | Baculovirus-Insect Cells |
| Synonyms | DLK,ZIP,ZIPK |
| Molecular Weight | The recombinant human DAPK3/GST chimera consists of 678 amino acids and predicts a molecular mass of 79 kDa. It migrates as an approximately 70 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 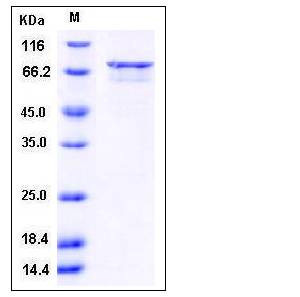 |
| Purity | > 85 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the full length of human DAPK3 (NP_001339.1) (Met 1-Arg 454) was fused with the GST tag at the N-terminus. |
| Bio-activity | The specific activity was determined to be 5 nmol/min/mg using MBP as substrate. |
| Research Area | Cardiovascular |Heart |Apoptosis |Other in Apoptosis |
| Formulation | Supplied as sterile 20mM Tris, 500mM NaCl, 10mM GSH, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Death-associated protein kinase 3, also known as DAP kinase 3, ZIP-kinase, DAPK3 and ZIPK, is a nucleus and cytoplasm protein which belongs to the protein kinase superfamily, CAMK Ser/Thr protein kinase family and DAP kinase subfamily. DAPK3 / ZIPK contains one protein kinase domain. It is a serine/threonine kinase which acts as a positive regulator of apoptosis. It phosphorylates histone H3 on 'Thr-11' at centromeres during mitosis. DAPK3 / ZIPK is a homodimer or forms heterodimers with ATF4. Both interactions require an intact leucine zipper domain and oligomerization is required for full enzymatic activity. It also binds to DAXX and PAWR, possibly in a ternary complex which plays a role in caspase activation. DAPK3 / ZIPK regulates myosin light chain phosphatase through phosphorylation of MYPT1 thereby regulating the assembly of the actin cytoskeleton, cell migration, invasiveness of tumor cells, smooth muscle contraction and neurite outgrowth. It is involved in the formation of promyelocytic leukemia protein nuclear body (PML-NB), one of many subnuclear domains in the eukaryotic cell nucleus, and which is involved in oncogenesis and viral infection. |
| Reference |
