Human DDOST / OST48 Protein
AGER1,CDG1R,OKSWcl45,OST,OST48,WBP1
- 100ug (NPP3797) Please inquiry
| Catalog Number | P12463-HNCE |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | AGER1,CDG1R,OKSWcl45,OST,OST48,WBP1 |
| Molecular Weight | The recombinant human DDOST consists of 387 amino acids and has a calculated molecular mass of 42.7 kDa. It migrates as an approximately 46 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Gly |
| SDS-PAGE | 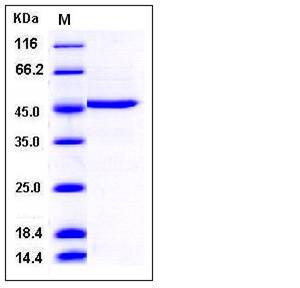 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human DDOST (P39656-1) extracellular domain (Ser 43-Pro 427) was expressed and purified, with additional two amino acids (Gly & Pro) at the N-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Protein Trafficking |Chaperones |Other Chaperones |
| Formulation | Lyophilized from sterile 50mM Tris, 150mM NaCl, pH 8.0 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | The enzyme oligosaccharyltransferase (dolichyl-diphosphooligosaccharide-protein glycosyltransferase) (DDOST), or 48-kDa subunit (OST48) is one of the catalytic subunits in this complex, exerts a typical type I membrane topology, containing a large luminal domain, a hydrophobic transmembrane domain and a short cytosolic peptide tail. DDOST/OST48 catalyzes the transfer of a high-mannose oligosaccharide (GlcNac2Man9Glc3) from a dolichol-linked oligosaccharide donor (dolichol-P-GlcNac2Man9Glc3) onto the asparagine acceptor site within an Asn-X-Ser/Thr consensus motif in nascent polypeptide chains across the membrane of the endoplasmic reticulum. The mammalian oligosaccharyltransferase (OST) is an oligomeric complex composed of three type I transmembrane proteins of the endoplasmic reticulum: ribophorin I (RI), ribophorin II (RII), and OST48. OST48 is not a glycoprotein and is not recognized by antibodies to either ribophorin. Like ribophorins I and II, OST48 was found to be an integral membrane protein, with the majority of the polypeptide located within the lumen of the endoplasmic reticulum (ER). OST48 does not show significant amino acid sequence homology to either ribophorin I or II. It had been found that only the luminal domain of RI contains ER retention information. The dilysine motif in OST48 functions as an ER localization motif because OST48 in which the two lysine residues are replaced by serine (OST48ss) is no longer retained in the ER and is found instead also at the plasma membrane. |
| Reference |
