Human DIM1 / TXNL4A Protein (His Tag)
BMKS,DIB1,DIM1,SNRNP15,TXNL4,U5-15kD
- 100ug (NPP2079) Please inquiry
| Catalog Number | P12434-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | BMKS,DIB1,DIM1,SNRNP15,TXNL4,U5-15kD |
| Molecular Weight | The recombinant human TXNL4A consists of 157 amino acids and has a calculated molecular mass of 18.6KDa. It migrates as an approximately 16 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 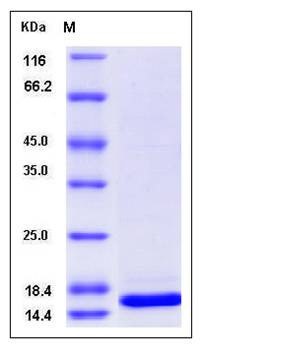 |
| Purity | > 94 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human TXNL4A (P83876) (Met 1-Tyr 142) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Epigenetics |DNA / RNA |RNA Processing |RNA splicing |
| Formulation | Lyophilized from sterile 50mM Tris, 150mM NaCl, pH 8.0 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | DIM1, also known as TXNL4A, is a member of the Dim protein family. The Dim protein family is composed of two classes, DIM1and Dim2, which share a common thioredoxin-like fold. They were originally identified for their role in cell cycle progression and have been found to interact with Prp6, an essential component of the spliceosome, which forms the bridge of U4/U6.U5-tri-snRNP. In spite of their biological and structural similarities, DIM1 and Dim2 proteins differ in many aspects. DIM1 bears distinctive structural motifs responsible for its interaction with other spliceosome components. Dim2 forms homodimers and contains specific domains required for its interactions with partners. This originality suggests that although both proteins are involved in pre-mRNA splicing, they are likely to be involved in different biological pathways. DIM1 interacts with HNRPF, HNRPH2, NEDD9/HEF1 and PQBP1/NPW38. It plays an essential role in pre-mRNA splicing. |
| Reference |
