Human DLL4 Protein (Fc Tag)
Delta-like 4,hdelta2
- 100ug (NPP3806) Please inquiry
| Catalog Number | P10171-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | Delta-like 4,hdelta2 |
| Molecular Weight | The recombinant human DLL4/Fc is a disulfide-linked homodimeric protein after removal of the signal peptide. The reduced monomer consists of 736 amino acids and predicts a molecular mass of 81 kDa. As a result of glycosylation, the rh DLL4/Fc monomer migrates as approximately 100-110 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Ser 27 |
| SDS-PAGE | 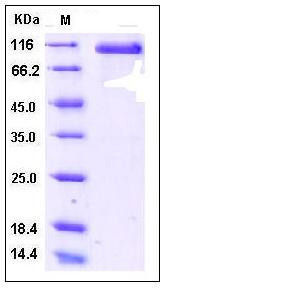 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Pro 524) of human DLL4 (NP_061947.1) pro-protein was expressed with the fused Fc region of human IgG1 at the C-terminus. |
| Bio-activity | 1. Measured by its binding ability in a functional ELISA. 2. Immobilized human DLL4 (cat: 10171-H02H) at 10 μg/mL (100 μL/well) can bind biotinylated mouse NOTCH1-his. The EC50 of biotinylated mouse NOTCH1-his is 40 ng/mL. 3. Measured by the ability of the immobilized protein to enhance BMP2-induced alkaline phosphatase activity in C3H10T1/2 mouse embryonic fibroblast cells. The ED50 for this effect is typically 1-8 µg/mL in the presence of 500 ng/mL recombinant human BMP2. |
| Research Area | Immunology |Signal Transduction |Notch Pathway |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Delta-like protein 4 (DLL4, Delta4), a type I membrane-bound Notch ligand, is one of five known Notch ligands in mammals and interacts predominantly with Notch 1, which has a key role in vascular development. Recent studies yield substantial insights into the role of DLL4 in angiogenesis. DLL4 is induced by vascular endothelial growth factor (VEGF) and acts downstream of VEGF as a 'brake' on VEGF-induced vessel growth, forming an autoregulatory negative feedback loop inactivating VEGF. DLL4 is downstream of VEGF signaling and its activation triggers a negative feedback that restrains the effects of VEGF. Attenuation of DLL4/Notch signaling results in chaotic vascular network with excessive branching and sprouting. DLL4 is widely distributed in tissues other than vessels including many malignancies. Furthermore, the molecule is internalized on binding its receptor and often transported to the nucleus. In pathological conditions, such as cancer, DLL4 is up-regulated strongly in the tumour vasculature. Blockade of DLL4-mediated Notch signaling strikingly increases nonproductive angiogenesis, but significantly inhibits tumor growth in preclinical mouse models. In preclinical studies, blocking of DLL4/Notch signaling is associated with a paradoxical increase in tumor vessel density, yet causes marked growth inhibition due to functionally defective vasculature. Thus, DLL4 blockade holds promise as an additional strategy for angiogenesis-based cancer therapy. |
| Reference |
