Human DPP7 / DPPII / DPP2 Protein (His Tag)
DPP2,DPPII,QPP
- 100ug (NPP2084) Please inquiry
| Catalog Number | P10748-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | DPP2,DPPII,QPP |
| Molecular Weight | The secreted recombinant human DPPII comprises 482 amino acids with a predicted molecular mass of 53.6 kDa. As a result of glycosylation, rh DPPII migrates as an approximately 60 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Gly 22 |
| SDS-PAGE | 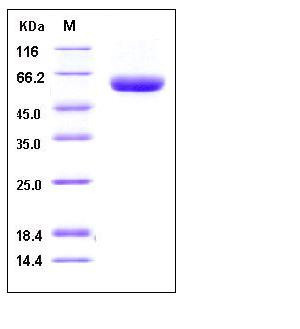 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human DPPII (NP_037511.2) (Met 1-Leu 492) with a C-terminal polyhistidine tag was expressed. |
| Bio-activity | |
| Research Area | Immunology |Innate Immunity |Complement System |MAC |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | DPP7 (dipeptidylpeptidase 7), also known as DPPII and DPP2, is a post-proline cleaving aminopeptidase expressed in quiescent lymphocytes. Dipeptidyl peptidases (DPPs) have post-proline dipeptidyl aminopeptidase activity, cleaving Xaa-Pro dipeptides from the N-termini of proteins. DPPs mediate regulatory activity of their substrates and have been linked to a variety of diseases including type 2 diabetes, obesity and cancer. DPPs can bind specific voltage-gated potassium channels and alter their expression and biophysical properties and may also influence T cells. DPP proteins include DPRP1, DPRP2, DPP3, DPP7, DPP10, DPPX and CD26. It localizes to lysosomes. DPP7 localizes to lysosomes and exists as a homodimer via its leucine zipper motif and is involved in the degradation of oligopeptides. In response to calcium release, it can be secreted in its active form. It is essential for lymphocyte survival, as the inhibition of DPP7 results in quiescent cell apoptosis. |
| Reference |
