Human E-Cadherin / CDH1 / E-cad / CD324 Protein (His Tag)
Arc-1,CD324,CDHE,E-cad,E-Cadherin,ECAD,LCAM,UVO
- 100ug (NPP3816) Please inquiry
| Catalog Number | P10204-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | Arc-1,CD324,CDHE,E-cad,E-Cadherin,ECAD,LCAM,UVO |
| Molecular Weight | The recombinant pro form of human E-Cad consists of 696 amino acids and has a calculated molecular mass of 77 kDa. As a result of glycosylation, the apparent molecular mass of rh E-Cad is approximately 80&95 kDa corresponding to the mature form and pro form respectively in SDS-PAGE under reducing conditions. |
| predicted N | Gln 23 & Asp 155 |
| SDS-PAGE | 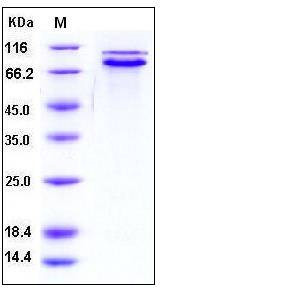 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain of human E-Cad precusor (NP_004351.1) (Met 1-Ile 707) was fused with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by the ability of the immobilized protein to support the adhesion of MCF-7 human breast adenocarcinoma cells. When cells are added to E-Cad coated plates (5 μg/mL, 100 μL/well), approximately 20%-40% of cells will adhere specifically after 90 minutes at 37 ℃. |
| Research Area | Cancer |Invasion microenvironment |Adhesion molecule |Cell adhesion |Cadherins |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Cadherins are calcium-dependent cell adhesion proteins which preferentially interact with themselves in a homophilic manner in connecting cells, and thus may contribute to the sorting of heterogeneous cell type. E-cadherin (E-Cad), also known as CDH1 and CD324, is a calcium-dependent cell adhesion molecule the intact function of which is crucial for the establishment and maintenance of epithelial tissue polarity and structural integrity. Mutations in CDH1 occur in diffuse type gastric cancer, lobular breast cancer, and endometrial cancer. In human cancers, partial or complete loss of E-cadherin expression correlates with malignancy. During apoptosis or with calcium influx, E-Cad is cleaved by the metalloproteinase to produce fragments of about 38 kDa (E-CAD/CTF1), 33 kDa (E-CAD/CTF2) and 29 kDa (E-CAD/CTF3), respectively. E-Cad has been identified as a potent invasive suppressor, as downregulation of E-cadherin expression is involved in dysfunction of the cell-cell adhesion system, and often correlates with strong invasive potential and poor prognosis of human carcinomas. |
| Reference |
