Human ECE-2 Protein (His Tag)
ECE2, hCG_2022032, KIAA0604, MGC17664, MGC2408, MGC78487
- 100ug (NPP3820) Please inquiry
| Catalog Number | P10207-H07H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | ECE2, hCG_2022032, KIAA0604, MGC17664, MGC2408, MGC78487 |
| Molecular Weight | The recombinant human ECE2 consists of 700 amino acids with the predicted molecular mass of 80.2 kDa. As a result of glycosylation, rhECE2 migrates as an approximately 120-125 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | His |
| SDS-PAGE | 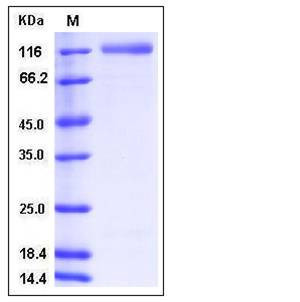 |
| Purity | > 93 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the ectodomain of human endothelin converting enzyme 2 isoform A (NP_055508.3) (Gly 199-Trp 883) was fused with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Cancer |Oncoprotein & suppressor & biomarker |Oncoprotein |Growth Factor & Receptor |Hormones |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Endothelin-converting enzyme 2, also known as ECE-2, is a metalloprotease that possesses many properties consistent with it being a neuropeptide-processing enzyme. Endothelin-converting enzymes (ECEs) are the key enzymes in the endothelin (ET) biosynthesis that catalyze the conversion of big ET, the biologically inactive precursor of mature ET. Two enzymes, termed ECE-1 and ECE-2, have been molecularly identified. ECE-2 is found primarily in neural tissues, with high levels of expression in midbrain, cerebellum, hypothalamus, frontal cortex and spinal cord and moderate levels in hippocampus and striatum. ECE-2 is strongly down-regulated in inferior parietal lobe from Alzheimer disease patients (at protein level). ECE-2 converts big endothelin-1 to endothelin-1. It is involved in the processing of various neuroendocrine peptides, including neurotensin, angiotensin I, substance P, proenkephalin-derived peptides, and prodynorphin-derived peptides. ECE-2 may limit beta-amyloid peptide accumulation in brain. It may also have methyltransferase activity. A comparison of residues around the cleavage site revealed that ECE-2 exhibits a unique cleavage site selectivity that is related to but distinct from that of ECE-1. |
| Reference |
