Human EDAR / DL Protein (His Tag)
DL,ECTD10A,ECTD10B,ED1R,ED3,ED5,EDA-A1R,EDA1R,EDA3,HRM1
- 100ug (NPP2095) Please inquiry
| Catalog Number | P11577-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | DL,ECTD10A,ECTD10B,ED1R,ED3,ED5,EDA-A1R,EDA1R,EDA3,HRM1 |
| Molecular Weight | The secreted recombinant human EDAR consists of 174 amino acids and has a predicted molecular mass of 19 kDa. The apparent molecular mass of the protein is approximately 30-40 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Glu 27 |
| SDS-PAGE | 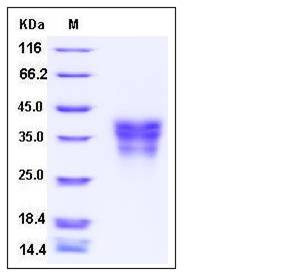 |
| Purity | > 96 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human EDAR (NP_071731.1) extracellular domain (Met 1-Ile 189) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Cancer |Signal transduction |Other Related Intracellular Topics |Cellular Senescence and Pathways in Aging |Apoptosis |Intracellular | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Tumor necrosis factor receptor superfamily member EDAR is a Single-pass type I membrane protein. Edar was expressed reiteratively in signaling centers regulating key steps in morphogenesis. activin signaling from mesenchyme induces the expression of the TNF receptor edar in the epithelial signaling centers, thus making them responsive to Wnt-induced ectodysplasin from the nearby ectoderm. This is the first demonstration of integration of the Wnt, activin, and TNF signaling pathways. Defects in EDAR are a cause of ectodermal dysplasia anhidrotic (EDA), also known ectodermal dysplasia hypohidrotic autosomal recessive (HED). Ectodermal dysplasia defines a heterogeneous group of disorders due to abnormal development of two or more ectodermal structures. EDA is characterized by sparse hair (atrichosis or hypotrichosis), abnormal or missing teeth and the inability to sweat due to the absence of sweat glands. |
| Reference |
