Human EGFR / HER1 / ErbB1 Protein (His Tag)
ERBB,ERBB1,HER1,mENA,NISBD2,PIG61
- 100ug (NPP1244) Please inquiry
| Catalog Number | P10001-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | ERBB,ERBB1,HER1,mENA,NISBD2,PIG61 |
| Molecular Weight | The recombinant human EGF receptor consists of 630 amino acids and has a calculated molecular mass of 69.8 kDa. As a result of glycosylation, the recombinant protein migrates as an approximately 110 kDa protein in SDS-PAGE under reducing conditions. |
| predicted N | Leu 25 |
| SDS-PAGE | 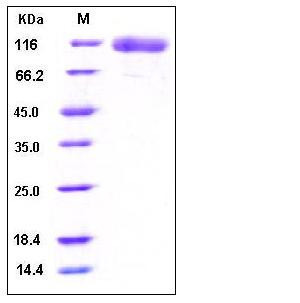 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Ser 645) of human EGFR (NP_005219) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to bind human EGF in a functional ELISA. |
| Research Area | Signaling |Signal Transduction |Protein Phosphorylation |Tyrosine Kinase |Receptor Tyrosine Kinases |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | As a member of the epidermal growth factor receptor (EGFR) family, EGFR protein is type I transmembrane glycoprotein that binds a subset of EGF family ligands including EGF, amphiregulin, TGF-α, betacellulin, etc. EGFR protein plays a crucial role in signaling pathway in the regulation of cell proliferation, survival and differentiation. Binding of a ligand induces EGFR protein homo- or heterodimerization, the subsequent tyrosine autophosphorylation and initiates various down stream pathways (MAPK, PI3K/PKB and STAT). In addition, EGFR signaling also has been shown to exert action on carcinogenesis and disease progression, and thus EGFR protein is proposed as a target for cancer therapy currently. |
| Reference |
