Human EPOR / Erythropoietin Receptor Protein (Fc Tag)
EPO-R
- 100ug (NPP2112) Please inquiry
| Catalog Number | P10707-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | EPO-R |
| Molecular Weight | The recombinant human EPOR/Fc is a disulfide-linked homodimeric protein after removal of the signal peptide. The reduced monomer consists of 460 amino acids and has a predicted molecular mass of 51.0 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhEPOR/Fc monomer is approximately 55-60 kDa due to glycosylation. |
| predicted N | Ala 25 |
| SDS-PAGE | 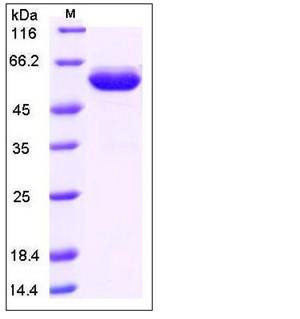 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met-Pro 250) of human erythropoietin receptor (NP_000112.1) precursor was expressed with the C-terminal fused Fc region of human IgG1. |
| Bio-activity | 1. Measured by its binding ability in a functional ELISA. Immobilized CD131 at 10 μg/ml (100 μl/well) can bind biotinylated recombinant human EPOR with a linear range of 0.16-4 μg/ml. 2. Measured by its ability to inhibit EPO-dependent proliferation of TF-1 human erythroleukemic cells. The ED50 for this effect is typically 4-16 ng/mL in the presence of 0.1 U/mL Recombinant Human EPO. |
| Research Area | Immunology |Signal Transduction |Transcription Factors and Regulators |HIF Transcription Factors |
| Formulation | Lyophilized from sterile PBS, 8% sucrose, 0.5% Tween-20, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Erythropoietin (EPO) is the major glycoprotein hormone regulator of mammalian erythropoiesis, and is produced by kidney and liver in an oxygen-dependent manner. The biological effects of EPO are mediated by the specific erythropoietin receptor (EPOR/EPO Receptor) on bone marrow erythroblasts, which transmits signals important for both proliferation and differentiation along the erythroid lineage. EPOR protein is a type â… single-transmembrane cytokine receptor, and belongs to the homodimerizing subclass which functions as ligand-induced or ligand-stabilized homodimers. EPOR signaling prevents neuronal death and ischemic injury. Recent studies have shown that EPO and EPOR protein may be involved in carcinogenesis, angiogenesis, and invasion. |
| Reference |
