Human ERH / DROER Protein (His Tag)
ERH
- 100ug (NPP2115) Please inquiry
| Catalog Number | P14483-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | ERH |
| Molecular Weight | The recombinant human ERH consists of 119 amino acids and predicts a molecular mass of 14.1 KDa. It migrates as an approximately 14 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | His |
| SDS-PAGE | 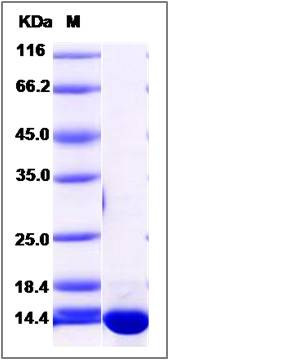 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mature form of human ERH (P84090) (Met1-Lys104) was expressed with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Epigenetics |Cell cycle |Chromosome Structure |Scaffold Protein |
| Formulation | Lyophilized from sterile 20mMTris, 0.1M NaCl, 1mM DTT, 20% glycerol pH 7.4. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | ERH(enhancer of rudimentary homolog) belongs to the E(R) family. It is expressed in all tissues examined. The monomeric structure of ERH comprises a single domain consisting of three α-helices and four β-strands, which is a novel fold. In the crystal structure, ERH assumes a dimeric structure, through interactions between the β-sheet regions. The formation of an ERH dimer is consistent with the results of analytical ultracentrifugation. ERH may have a role in the cell cycle. The Drosophila protein ERH is a small protein of 104 amino acids. It has been found to be an enhancer of the rudimentary gene, involved in pyrimidine biosynthesis. From an evolutionary point of view, ERH is highly conserved and has been found to exist in probably all multicellular eukaryotic organisms. ERH interacts with POLDIP3. |
| Reference |
