Human ERP27 Protein (Fc Tag)
C12orf46,PDIA8
- 100ug (NPP3850) Please inquiry
| Catalog Number | P13256-H05H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | C12orf46,PDIA8 |
| Molecular Weight | The recombinant human ERP27/mFc is a disulfide-linked homodimer. The reduced monomer comprises 478 amino acids and has a predicted molecular mass of 53.7 kDa. The apparent molecular mass of the protein is approximately 59 in SDS-PAGE under reducing conditions due to glycosylation. |
| predicted N | Glu 26 |
| SDS-PAGE | 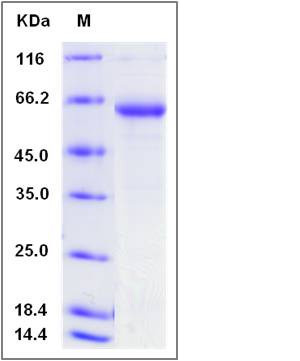 |
| Purity | > 84 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human ERP27 (Q96DN0) (Glu26-Pro269) was fused with Fc region of mouse IgG at the C-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Protein Trafficking |ER Proteins |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | ERP27 contains 1 thioredoxin domain and is a noncatalytic member of the protein disulfide isomerase family. Protein disulfide isomerases (PDIs) constitute a family of structurally related enzymes which catalyze disulfide bonds formation, reduction, or isomerization of newly synthesized proteins in the lumen of the endoplasmic reticulum (ER). They act also as chaperones, and are, therefore, part of a quality-control system for the correct folding of the proteins in the same subcellular compartment. PDI has been found to have moderate effects (25-fold) on the rate of oxidative folding of proteins in vitro. Recombinant Human Protein Disulfide Isomerase is involved in disulphide-bond formation and isomerization, as well as the reduction of disulphide bonds in proteins. Recombinant PDI has been found to have moderate effects (25-fold) on the rate of oxidative folding of proteins in vitro. ERP27 is a widely expressed protein which localizes to the ER and may act as a protease, protein disulfide isomerase, thiol-disulfide oxidase or phospholipase. ERP27 doesn't contain a CXXC active site motif indicating that it is a catalytically redox-inactive member of the protein disulfide isomerase family. |
| Reference |
