Human Endoglin / CD105 / ENG Protein (Fc Tag)
END,HHT1,ORW1
- 100ug (NPP3823) Please inquiry
| Catalog Number | P10149-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | END,HHT1,ORW1 |
| Molecular Weight | The recombinant human CD105/Fc chimera is a disulfide-linked homodimeric protein. The reduced monomer consists of 799 amino acids and has a predicted molecular mass of 87.5 kDa. As a result of glycosylation, rhCD105/Fc monomer migrates as an approximately 115-120 kDa protein in SDS-PAGE under reducing conditions. |
| predicted N | Glu 26 |
| SDS-PAGE | 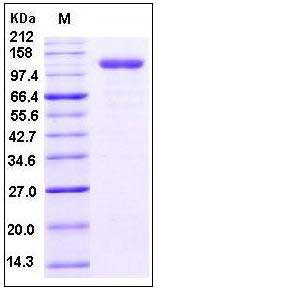 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Gly 586) of human CD105 (NP_001108225.1) precursor was expressed with C-terminal fused human IgG1 Fc region. |
| Bio-activity | 1. Measured by its ability to bind biotinylated Human TGFRB1 in a functional ELISA. 2. Measured by its ability to bind biotinylated Human ALK1-Fc (P10066-H02H) in functional Elisa. |
| Research Area | Immunology |Cytokines & Growth Factors |Cytokine & Receptor |Transforming Growth Factor Beta (TGF-beta) Superfamily |Transforming Growth Factor Beta (TGF-beta) Family |TGF-beta Family Receptor | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Endoglin, also known as CD105, is a type I homodimeric transmembrane glycoprotein with a large, disulfide-linked, extracellular region and a short, constitutively phosphorylated cytoplasmic tail. Endoglin contains an RGD tripeptide which is a key recognition structure in cellular adhesion,,suggesting a critical role for endoglin in the binding of endothelial cells to integrins and/or other RGD receptors. Endoglin is highly expressed on vascular endothelial cells, chondrocytes, and syncytiotrophoblasts of term placenta. It is also found on activated monocytes, mesenchymal stem cells and leukemic cells of lymphoid and myeloid lineages. As an accessory receptor for the TGF-β superfamily ligands, endoglin binds TGF-β1 and TGF-β3 with high affinity not by itself but by associating with TGF-β type I I receptor (TβRII) and activates the downstream signal pathways. In addition, in human umbilical vein endothelial cells, ALK-1 is also a receptor kinase for endoglin threonine phosphorylation, and mutations in either of the two genes result in the autosomal-dominant vascular dysplasia, hereditary hemorrhagic telangiectasia (HHT). Endoglin has been regarded as a powerful biomarker of neovascularization, and is associated with several solid tumor types. |
| Reference |
