Human EphB2 Protein (His Tag)
CAPB,DRT,EK5,EPHT3,ERK,Hek5,PCBC,Tyro5
- 100ug (NPP3839) Please inquiry
| Catalog Number | P10762-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CAPB,DRT,EK5,EPHT3,ERK,Hek5,PCBC,Tyro5 |
| Molecular Weight | The recombinant human EphB2 consists of 536 amino acids and has a calculated molecular mass of 59.7 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhEphB2 is approximately 70-75 kDa due to glycosylation. |
| predicted N | Val 19 |
| SDS-PAGE | 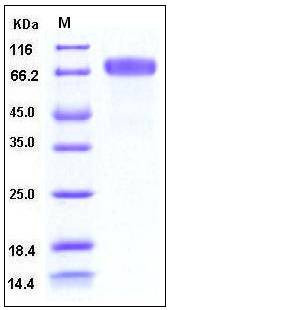 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Leu 543) of human EphB2 (NP_059145.2) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | Measured by its ability to bind recombinant human EphrinB2 / Fc chimera in a functional ELISA. |
| Research Area | Signaling |Signal Transduction |Growth Factor & Receptor |Receptor Tyrosine Kinase (RTK) |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Ephrin type-B receptor 2, also known as EphB2, belongs to the ephrin receptor subfamily of the protein-tyrosine kinase family which 16 known receptors (14 found in mammals) are involved: EPHA1, EPHA2, EPHA3, EPHA4, EPHA5, EPHA6, EPHA7, EPHA8, EPHA9, EPHA10, EPHB1, EPHB2, EPHB3, EPHB4, EPHB5, EPHB6. EphB2 receptor tyrosine kinase phosphorylates syndecan-2 and that this phosphorylation event is crucial for syndecan-2 clustering and spine formation. The Eph family of receptor tyrosine kinases (comprising EphA and EphB receptors) has been implicated in synapse formation and the regulation of synaptic function and plasticity6. Ephrin receptors are components of cell signalling pathways involved in animal growth and development, forming the largest sub-family of receptor tyrosine kinases (RTKs). Ligand-mediated activation of Ephs induce various important downstream effects and Eph receptors have been studied for their potential roles in the development of cancer. EphB receptor tyrosine kinases are enriched at synapses, suggesting that these receptors play a role in synapse formation or function. We find that EphrinB binding to EphB induces a direct interaction of EphB with NMDA-type glutamate receptors. This interaction occurs at the cell surface and is mediated by the extracellular regions of the two receptors, but does not require the kinase activity of EphB. |
| Reference |
