Human EphB6 / EphB6 Protein (Fc Tag)
HEP
- 100ug (NPP3841) Please inquiry
| Catalog Number | P10197-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | HEP |
| Molecular Weight | The recombinant mature human EphB6/Fc chimera is a disulfide linked homodimeric protein. Each monomer consists of 801 amino acids and has a calculated molecular mass of 86.5 kDa. In SDS-PAGE under reducing conditions, rhEphB6/Fc monomer migrates with an apparent molecular mass of approximately 100-110 kDa due to glycosylation. |
| predicted N | Leu 17 |
| SDS-PAGE | 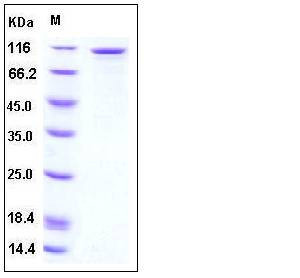 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | The extracellular domain (Met 1-Ser 579) of human EphB6 (NP_004436.1) precursor was fused with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | 1. Measured by its binding ability in a functional ELISA. 2. Immobilized recombinant human EphrinB1 at 10 μg/ml (100 μl/well) can bind human EphB6 with a linear range of 0.16-4 μg/ml. 3. Immobilized recombinant human EphrinB2 at 10 μg/ml (100 μl/well) can bind human EphB6 with a linear range of 1.28-32 ng/ml. |
| Research Area | Signaling |Signal Transduction |Growth Factor & Receptor |Ephrin & Eph Receptor |Eph Receptor |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Ephrins are divided into the ephrin-A (EFNA) class and the ephrin-B (EFNB) class based on their structures and sequence relationships. Ephrin receptors make up the largest subgroup of the receptor tyrosine kinase (RTK) family. EphB6 is an unusual Eph receptor, lacking catalytic capacity due to alterations in its kinase domain. Interestingly, increased metastatic activity is associated with reduced EphB6 receptor expression in several tumor types, including breast cancer. This emphasizes the potential of EphB6 to act as a suppressor of cancer aggressiveness. EphB6 suppress cancer invasiveness through c-Cbl-dependent signaling, morphologic changes, and cell attachment and indicate that EphB6 may represent a useful prognostic marker and a promising target for therapeutic approaches. EphB6 can both positively and negatively regulate cell adhesion and migration, and suggest that tyrosine phosphorylation of the receptor by an Src family kinase acts as the molecular switch for the functional transition. In addition, Ephrin-B2 may be a physiological ligand for the EphB6 receptor. |
| Reference |
