Human Ephrin-B1 / EFNB1 Protein (His & Fc Tag)
CFND,CFNS,EFB1,EFL3,Elk-L,EPLG2,LERK2
- 100ug (NPP3844) Please inquiry
| Catalog Number | P10894-H03H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CFND,CFNS,EFB1,EFL3,Elk-L,EPLG2,LERK2 |
| Molecular Weight | The recombinant human EFNB1/Fc chimera is a disulfide-linked homodimeric protein. The reduced monomer consists of 458 amino acids and predicts a molecular mass of 51.2 KDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of the protein is approximately 64 and 36 KDa due to glycosylation. |
| predicted N | Leu 28 |
| SDS-PAGE | 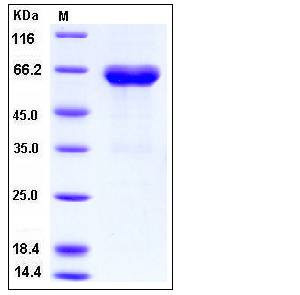 |
| Purity | > (79.7+18.0) % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human EFNB1 (NP_004420.1) extracellular domain (Met 1-Gly 232) was was fused with the C-terminal polyhistidine-tagged Fc region of human IgG1 at the C-terminus. |
| Bio-activity | Measured by its binding ability in a functional ELISA . Immobilized mouse EphB3 at 2 μg/ml (100 μl/well) can bind human EFNB1 Fc chimera with a linear ranger of 1.56-25 ng/ml. |
| Research Area | Neuroscience |Cell Type Marker in Neurodevelopment |Neuronal Cell Markers |Growth Cone |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Ephrin-B1 also known as EFNB1, is a member of the ephrin family. The transmembrane- associated ephrin ligands and their Eph family of receptor tyrosine kinases are expressed by cells of the SVZ. Eph/ephrin interactions are implicated in axon guidance, neural crest cell migration, establishment of segmental boundaries, and formation of angiogenic capillary plexi. Eph receptors and ephrins are divided into two subclasses, A and B, based on binding specificities. Ephrin subclasses are further distinguished by their mode of attachment to the plasma membrane: ephrin-A ligands bind EphA receptors and are anchored to the plasma membrane via a glycosylphosphatidylinositol (GPI) linkage, whereas ephrin-B ligands bind EphB receptors and are anchored via a transmembrane domain. An exception is the EphA4 receptor, which binds both subclasses of ephrins. EphrinB1 and B class Eph receptors provide positional cues required for the normal morphogenesis of skeletal elements. Another malformation, preaxial polydactyly, was exclusively seen in heterozygous females in which expression of the X-linked ephrinB1 gene was mosaic, so that ectopic EphB-ephrinB1 interactions led to restricted cell movements and the bifurcation of digital rays. |
| Reference |
