Human FAP / Seprase Protein (His Tag)
DPPIV,DPPIVA,FAPA,Fibroblast Activation Protein alpha,SIMP
- 100ug (NPP3865) Please inquiry
| Catalog Number | P10464-H07H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | DPPIV,DPPIVA,FAPA,Fibroblast Activation Protein alpha,SIMP |
| Molecular Weight | The recombinant human FAP consists of 751 amino acids and predicts a molecular mass of 87.2 kDa. As a result of glycosylation, rh FAP migrates as approximately 90 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | His |
| SDS-PAGE | 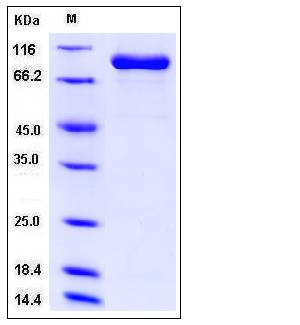 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human FAP isoform 1 (Q12884-1) extracellular domain (Leu 26-Asp 760) was fused with the polyhistidie-tag at the N-terminus. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Inflammatory Cytokines & Chemoki and Receptors |Chemokines and Receptors |CC Chemokines and Receptors |
| Formulation | Lyophilized from sterile 25mM Tris, 250mM NaCl, pH 8.2 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Seprase, also known as 170 kDa melanoma membrane-bound gelatinase , Fibroblast activation protein alpha, Integral membrane serine protease and FAP, is a single-pass type II membrane protein which belongs to the peptidase S9B family. Seprase / FAP is found in cell surface lamellipodia, invadopodia and on shed vesicles. Seprase / FAP appears to act as a proteolytically active 170-kDa dimer, consisting of two 97-kDa subunits. It is a member of the group type II integral serine proteases, which includes dipeptidyl peptidase IV ( DPPIV / CD26 ) and related type II transmembrane prolyl serine peptidases, which exert their mechanisms of action on the cell surface. Seprase / FAP colocalized with DPP4 in invadopodia and lamellipodia of migratory activated endothelial cells in collagenous matrix. Seprase / FAP colocalized with DPP4 on endothelial cells of capillary-like microvessels but not large vessels within invasive breast ductal carcinoma. DPP4 and seprase exhibit multiple functions due to their abilities to form complexes with each other and to interact with other membrane-associated molecules. In association with DPP4, Seprase / FAP is involved in the pericellular proteolysis of the extracellular matrix (ECM), the migration and invasion of endothelial cells into the ECM. Seprase / FAP has a dual function in tumour progression. The proteolytic activity of Seprase has been shown to promote cell invasiveness towards the ECM and also to support tumour growth and proliferation. Seprase / FAP may have a role in tissue remodeling during development and wound healing, and may contribute to invasiveness in malignant cancers. |
| Reference |
